hardness → tvrdoća
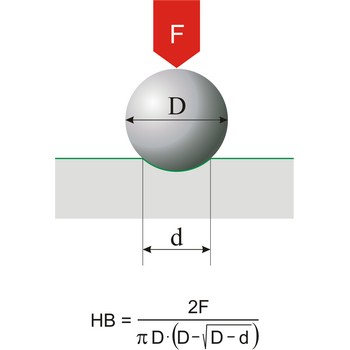
Hardness is the resistance of a material to deformation of an indenter of specific size and shape under a known load. This definition applies to all types of hardness scales except Mohs scale, which is a based on the concept of scratch hardness and is used chiefly for minerals. The most generally used hardness scales are Brinell (for cast iron), Rockwell (for sheet metal and heat-treated steel), Knoop (for metals).
heat capacity → toplinski kapacitet
Heat capacity is defined in general as dQ/dT, where dQ is the amount of heat that must be added to a system to increase its temperature by a small amount dT. The heat capacity at a constant pressure is Cp = (∂H/∂T)p; that at a constant volume is CV = (∂E/∂T)V, where H is enthalpy, E is internal energy, p is pressure, V is volume, and T is temperature. An upper case C normally indicates the molar heat capacity, while a lower case c is used for the specific (per unit mass) heat capacity.
hemiacetal → poluacetal
Hemiacetals are organic compounds having the general formula R2C(OH)OR’ (R’ ≠ H), derived from aldehydes or ketones by formal addition of an alcohol to the carbonyl group. Hemiacetals are generally unstable compounds. In some cases however, stable cyclic hemiacetals can be readily formed, especially when 5- and 6-membered rings are possible. In this case an intramolecular OH group reacts with the carbonyl group. Glucose and many other aldoses exist as cyclic hemiacetals whereas fructose and similar ketoses exist as cyclic hemiketals. Originally, the term was confined to derivatives of aldehydes (one R = H), but it now applies equally to derivatives of ketones (neither R = H).
Moh’s scale → Mohsova skala
Mohs’ scale of mineral hardness characterises the scratch resistance of various minerals through the ability of a harder material to scratch a softer. It was created by the German mineralogist Friedrich Mohs (1773-1839). Mohs based the scale on the ten readily available minerals.
| Hardness | Mineral |
|---|---|
| 1 | talc (Mg3Si4O10(OH)2) |
| 2 | gypsum (CaSO4·2H2O) |
| 3 | calcite (CaCO3) |
| 4 | fluorite (CaF2) |
| 5 | apatite (Ca5(PO4)3(OH-,Cl-,F-)) |
| 6 | orthoclase feldspar (KAlSi3O8) |
| 7 | quartz (SiO2) |
| 8 | topaz (Al2SiO4(OH-,F-)2) |
| 9 | corundum (Al2O2) |
| 10 | diamond (C) |
molar mass → molarna masa
Molar mass is the mass of one mole of a substance.
It is normally expressed in units of g mol-1, in which case its numerical value is identical with the relative molecular mass.
monosaccharide → monosaharid
Monosaccharides are carbohydrates, with the general formula Cn(H2O)n, that cannot be decomposed to a simpler carbohydrates by hydrolysis.
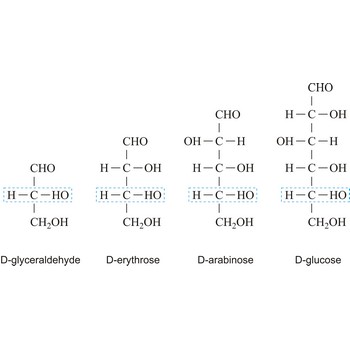
Depending on whether the molecule contains an aldehyde group (-CHO) or a ketone group (-CO-) monosaccharide can be a polyhydroxy aldehyde (aldose) or a polyhydroxy ketone (ketose). These aldehyde and ketone groups confer reduction properties on monosaccharides. They are also classified according to the number of carbon atoms they contain: trioses have three carbon atoms, tetroses four, pentoses five, hexoses six, heptoses seven, etc. These two systems of classification are often combined. For example, a six-carbon polyhydroxy aldehyde such as D-glucose is an aldohexose, whereas a six-carbon polyhydroxy ketone such as D-fructose is a ketohexose.
The notations D and L are used to describe the configurations of carbohydrates. In Fischer projections of monosaccharides, the carbonyl group is always placed on top (in the case of aldoses) or as close to the top as possible (in the case of ketoses). If the OH group attached to the bottom-most asymmetric carbon (the carbon that is second from the bottom) is on the right, then the compound is a D-sugar. If the OH group is on the left, then the compound is an L-sugar. Almost all sugars found in nature are D-sugars.
Monosaccharides can exist as either straight-chain or ring-shaped molecules. During the conversion from straight-chain form to cyclic form, the carbon atom containing the carbonyl oxygen, called the anomeric carbon, becomes a chiral center with two possible configurations (anomers), α and β. When the stereochemistry of the first carbon matches the stereochemistry of the last stereogenic center the sugar is the α-anomer when they are opposite the sugar is the β-anomer.
polymer → polimer
Polymer is a substance composed of molecules of high relative molecular mass (molecular weight), the structure of which essentially comprises the multiple repetition of units derived, actually or conceptually, from molecules of low relative molecular mass (monomers). In most cases the number of monomers is quite large and is often not precisely known. A single molecule of a polymer is called a macromolecule. Polystyrene is light solid material obtained by polymerisation of styrene (vinyl benzene).
refractometer → refraktometar
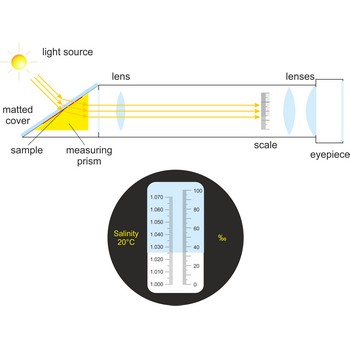
Refractometer is an optical device used from measurement of refractive index. A refractometer takes advantage of the fact that light bends as it passes through different materials. It can be used to measure the salinity of water or the amount of sugar in fresh grapes. Refractometers are available with or without automatic temperature compensation (ATC).
When using a conventional saltwater refractometer, a sample is placed on an optical prism in the sample window. As light shines through the sample, it is bent according to the salinity of the water, and casts a shadow on the scale that is visible through the eyepiece. Salinity is read directly through the eyepiece.
resonance → rezonancija
Resonance is a stabilising quality of certain molecules that can be represented by considering the electron distribution in an ion or molecule as a composite of two or more forms, in those cases where a single form is an inadequate representation; for example, benzene and the carbonate ion. A various canonical structures can be drawn to show how electron delocalisation will explain the discrepancy, the difference in electron density
tin → kositar
Tin has been known since ancient times. The origin of the name comes from the Latin word stannum meaning tin. It is silvery-white, soft, malleable and ductile metal. Exposed surfaces form oxide film. Resists oxygen and water. Dissolves in acids and bases. Organic tin compounds may be highly toxic. Tin is principally found in the ore cassiterite (SnO2) and stannine (Cu2FeSnS4). Used as a coating for steel cans since it is non-toxic and non-corrosive. Also in solder (33 %Sn:67 %Pb), bronze (20 %Sn:80 %Cu) and pewter. Stannous fluoride (SnF2), a compound of tin and fluorine is used in some toothpaste.
Citing this page:
Generalic, Eni. "Ventas de casa en la ciudadela orellana guayaquil." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table