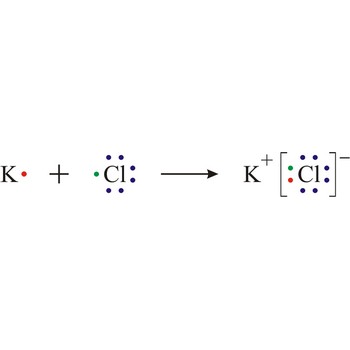ion exchanger → ionski izmjenjivač
Ion-exchanger is a solid or liquid material containing ions that are exchangeable with other ions with a like charge that are present in a solution in which the material is insoluble. Ion-exchange resins consist of various copolymers having a cross-linked three-dimensional structure to which ionic groups have been attached.
ionisation → ionizacija
Ionisation is the process of producing ions. Certain molecules ionise in a solution; for example, acids ionise when dissolved in water.
Electron transfer also causes ionisation in certain reactions, for example sodium and chlorine react by transfer of a valence electron from the sodium atom to the chlorine atom to form the ions that constitute a sodium chloride crystal.
Kjeldahl’s method → Kjeldahlov postupak
Kjeldahl’s method is an analytical method for determination of nitrogen in certain organic compounds. The method was developed by the Danish chemist Johan Kjeldahl (1849-1900).
It involves addition of a small amount of anhydrous potassium sulphate to the test compound, followed by heating the mixture with concentrated sulphuric acid, often with a catalyst such as copper sulphate. As a result ammonia is formed. After alkalyzing the mixture with sodium hydroxyde, the ammonia is separated by distillation, collected in standard acid, and the nitrogen determined by back-titration.
- Kjeldahl flask for decomposition (500 ml – macro or 100 ml - micro)
- funnel for alkaline solution
- Wagner tube (drop catcher)
- condenser
- absorption flask with known volume of standard acid
lactose → laktoza
Lactose (milk sugar) is a disaccharide comprising one glucose molecule linked to a galactose molecule by an β(1→4)-glycosidic linkage. Lactose has a beta acetal. Lactose is manufactured by the mammary gland and occurs only in milk (from 4 % to 7 %). Lactose intolerance is a common medical condition that results in diarrhea, abdominal pain, and flatulence and is caused by reduced or absent activity of enzyme lactase.
Like cellobiose and maltose, lactose is a reducing sugar. All reducing sugar undergo mutarotation in aqueous solution. The equilibrium mixture at 20 °C is composed of 62.7 % β-lactose (β-D-galactopyranosyl-(1→4)-β-D-glucopyranose) and 37.3 % α-lactose (β-D-galactopyranosyl-(1→4)-α-D-glucopyranose).
Lewis structure → Lewisova struktura
Lewis structure is the representation of the electron arrangement in atoms, ions, or molecules by showing the valence electrons as dots placed around the symbols for the elements.
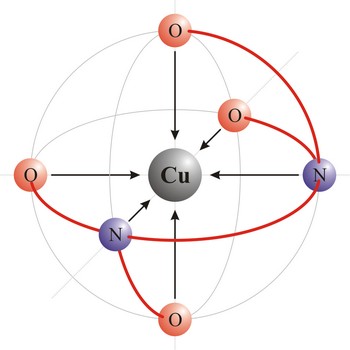
ligand → ligand
Ligand is an ion (F-, Cl-, Br-, I-, S2-, CN-, NCS-, OH-, NH2-) or molecule (NH3, H2O, NO, CO) that donates a pair of electrons to a metal atom or ion in forming a coordination complex. The main way of classifying ligands is by the number of points at which they are attached to, or bound to, the metal center. This is the denticity. Ligands with one potential donor atom are monodentate. Polydentate ligand is a ligand that is attached to a central metal ion by bonds from two or more donor atoms. Ligands with more than one potential donor atom are known as ambidentate, such as the thiocyanate ion, NCS-, which can bind to the metal center with either the nitrogen or sulphur atoms. Chelating ligands are those polydentate ligands which can form a ring including the metal atom.
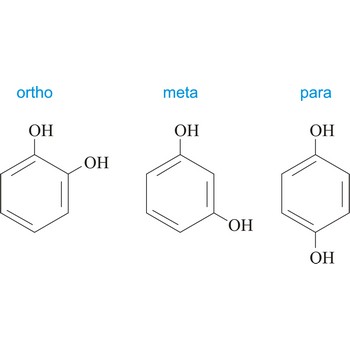
meta position → meta položaj
Meta position in organic chemistry is the one in which there are two same functional groups tied to a ring of benzene in position 1 and 3. The abbreviation m- is used, for example, m-Hydroquinone is 1,3-dihydroxybenzene.
molecular shape → oblik molekule
Molecular shape is the three dimensional arrangement of atoms in space around a central atom. The molecular formula of a substance does not give an indication of its shape. For example, CO2 is a linear molecule, but SO2 is angular.
The three-dimensional shapes of many small molecules can be predicted by applying the valence shell electron pair repulsion theory (VSEPR). When atoms combine to form molecules, pairs of valence electrons arrange themselves as far from each other as possible. Another way to characterize molecular shape is in terms of hybrid orbitals.
Citing this page:
Generalic, Eni. "Valentna veza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table