dehydrogenation → dehidrogenacija
Dehydrogenation is a chemical reaction in which hydrogen is removed from a compound. Dehydrogenation of organic compounds converts single carbon-carbon bonds into double bonds. It is usually affected by means of a metal catalyst or in biological systems by enzyme dehydrogenases.
carbohydrate → ugljikohidrat
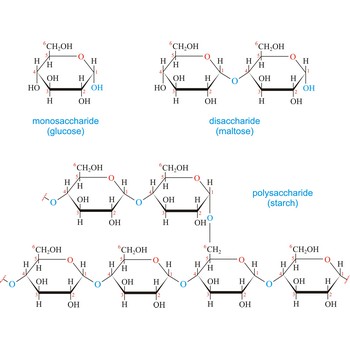
Carbohydrates (often called carbs for short) are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis. They are also known as saccharides, a term derived from the Latin word saccharum for sugar. Carbohydrates are the most abundant class of compounds in the biological world, making up more than 50 % of the dry weight of the Earth’s biomass. Every type of food we eat can have its energy traced back to a plant. Plants use carbon dioxide and water to make glucose, a simple sugar, in photosynthesis. Other carbohydrates such as cellulose and starch are made from the glucose. Light from the sun is absorbed by chlorophyll and this is converted to the energy necessary to biosynthesize carbohydrates
The term carbohydrate was applied originally to monosaccharides, in recognition of the fact that their empirical composition can be expressed as Cx(H2O)y. Later structural studies revealed that these compounds were not hydrates but the term carbohydrate persists.
Carbohydrates are generally classed as either simple or complex. Simple sugars, or monosaccharides, are carbohydrates that can’t be converted into smaller subunits by hydrolysis. Complex carbohydrates are made of two (disaccharides) or more (oligosaccharides, polysaccharides) simple sugars linked together by acetal (glycosidic) bonds and can be split into the former by hydrolysis.
cement → cement
Cement is any various substances used for bonding or setting to a hard material. Portland cement is a mixture of calcium silicates and aluminates made by heating limestone (CaCO3) with clay (containing aluminosilicates) in a kiln. The product is ground to a fine powder. When mixed with water it settles in a few hours and then hardens over a longer period of time due to the formation of hydrated aluminates and silicates.
electromagnetic wave → elektromagnetski val
All matter absorbs and emits radiation covering a broad band of frequencies and wavelengths. Thus electromagnetic radiation has the velocity of light (2.998×108 ms-1) and arises from the vibrating electric charges in atoms and bonds. The range of wavelengths, also known as the electromagnetic spectrum, is shown below:
electron pair → elektronski par
Electron pair is two electrons within one orbital with opposite spins responsible for a chemical bond.
Fermi level → Fermijev nivo
Fermi level is the highest energy of occupied states in a solid at zero temperature. The Fermi level in conductors lies in the conduction band, in insulators it lies in the valence band, and in semiconductors it falls in the gap between the conduction band and the valence band. It was named after the Italian physicst Enrico Fermi (1901 - 1954).
Gaussian system of units → Gaussov sustav jedinica
Gaussian system of units is a hybrid system used in electromagnetic theory, which combines features of both the electrostatic cgs subsystem (esu) and electromagnetic cgs subsystem (emu). With three base units, it uses em units in magnetism and es units in electrostatics. This involves using the constant c (the velocity of light in vacuum) to interrelate these sets of units.
Grignard reagent → Grignardov reagens
Grignard reagents are organomagnesium halides, RMgX, having a carbon- magnesium bond (or their equilibrium mixtures in solution with R2Mg + MgX2).
Citing this page:
Generalic, Eni. "Valence bond theory." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table