catalytic cracking → katalitčko krekiranje
Catalytic cracking is a petroleum refining process in which heavy-molecular weight hydrocarbons are broken up into light hydrocarbon molecules by the application of heat and pressure in the presence of a catalyst.
cracking → krekiranje
Cracking is the process whereby heavy molecules of petroleum or crude oil are broken down into hydrocarbons of lower molecular weight (especially in the oil-refining process).
halocarbon → halogenirani ugljikovodik
Halocarbon is a compound containing no elements other than carbon, one or more halogens, and sometimes hydrogen. The simplest are compounds such as tetrachloromethane (CCl4), tetrabromomethane (CBr4), etc. The lower members of the various homologous series are used as refrigerants, propellant gases, fireextinguishing agents, and blowing agents for urethane foams. When polymerized, they yield plastics characterized by extreme chemical resistance, high electrical resistivity, and good heat resistance.
hydrophobic interaction → hidrofobne interakcije
Hydrophobic interaction is the tendency of hydrocarbons (or of lipophilic hydrocarbon-like groups in solutes) to form intermolecular aggregates in an aqueous medium, and analogous intramolecular interactions. The name arises from the attribution of the phenomenon to the apparent repulsion between water and hydrocarbons. Use of the misleading alternative term hydrophobic bond is discouraged.
unsaturated hydrocarbon → nezasićeni ugljikovodik
Unsaturated hydrocarbons are organic compounds containing double (alkenes) or triple (alkynes) bonds in their molecules.
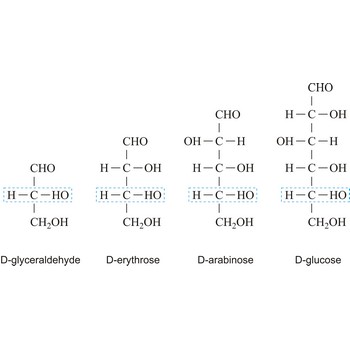
monosaccharide → monosaharid
Monosaccharides are carbohydrates, with the general formula Cn(H2O)n, that cannot be decomposed to a simpler carbohydrates by hydrolysis.
Depending on whether the molecule contains an aldehyde group (-CHO) or a ketone group (-CO-) monosaccharide can be a polyhydroxy aldehyde (aldose) or a polyhydroxy ketone (ketose). These aldehyde and ketone groups confer reduction properties on monosaccharides. They are also classified according to the number of carbon atoms they contain: trioses have three carbon atoms, tetroses four, pentoses five, hexoses six, heptoses seven, etc. These two systems of classification are often combined. For example, a six-carbon polyhydroxy aldehyde such as D-glucose is an aldohexose, whereas a six-carbon polyhydroxy ketone such as D-fructose is a ketohexose.
The notations D and L are used to describe the configurations of carbohydrates. In Fischer projections of monosaccharides, the carbonyl group is always placed on top (in the case of aldoses) or as close to the top as possible (in the case of ketoses). If the OH group attached to the bottom-most asymmetric carbon (the carbon that is second from the bottom) is on the right, then the compound is a D-sugar. If the OH group is on the left, then the compound is an L-sugar. Almost all sugars found in nature are D-sugars.
Monosaccharides can exist as either straight-chain or ring-shaped molecules. During the conversion from straight-chain form to cyclic form, the carbon atom containing the carbonyl oxygen, called the anomeric carbon, becomes a chiral center with two possible configurations (anomers), α and β. When the stereochemistry of the first carbon matches the stereochemistry of the last stereogenic center the sugar is the α-anomer when they are opposite the sugar is the β-anomer.
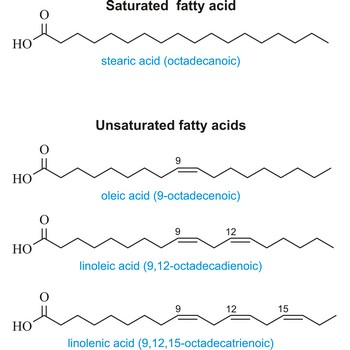
saturated fatty acid → zasićena masna kiselina
Saturated fatty acid is a fatty acid carrying the maximum possible number of hydrogen atoms (It doesn’t have any double bounds in the alkyl chain). The most important of these are:
| Butyric (butanoic acid) | CH3(CH2)2COOH |
| Lauric (dodecanoic acid) | CH3(CH2)10COOH |
| Myristic (tetradecanoic acid) | CH3(CH2)12COOH |
| Palmitic (hexadecanoic acid) | CH3(CH2)14COOH |
| Stearic (octadecanoic acid) | CH3(CH2)16COOH |
| Arachidic (eicosanoic acid) | CH3(CH2)18COOH |
Citing this page:
Generalic, Eni. "Ugljikovodici." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table