hybrid orbital → hibridne orbitale
Hybrid orbital is an orbital created by mixing together atomic orbitals to form an equal number of new hybrid atomic orbitals. For example, a common hybridization is sp3 where s orbital combine with a three p orbitals to form four new orbitals. After hybridization, all hybrid orbitals have the same energy, lower than p orbitals, but higher than s orbitals.
sp hybrid orbital → sp hibridna orbitala
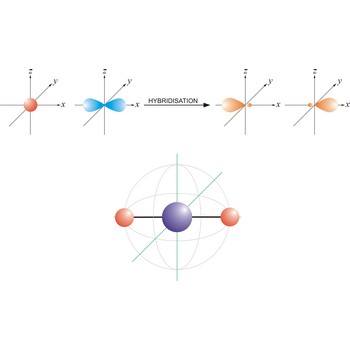
An sp hybrid orbital is an orbital formed by the linear combination of one s and one p orbital of comparable energy (such 2s and 2p orbitals) on a same atom. The two sp hybrid orbitals are aligned in a straight line in opposite direction (bond angles are 180°). The remaining two p orbitals are at right angles to one another and to the line formed by the two sp orbitals.
sp2 hybrid orbital → sp2 hibridna orbitala
An sp2 hybrid orbital is an orbital formed by the linear combination of one s and two p orbitals of comparable energy (such 2s and 2p orbitals) on a same atom. The three sp2 hybrid orbitals lie in a plane with angle of 120°. The remaining p orbital remains unchanged and is perpendicular to the plane of the three sp2 orbitals.
sp3 hybrid orbital → sp3 hibridna orbitala
An sp3 hybrid orbital is an orbital formed by the linear combination of one s and three p orbitals of comparable energy (such 2s and 2p orbitals) on a same atom. The four sp3 hybrid orbitals point toward the corners of a regular tetrahedron with the bond angle of 109.5°.
hybridization → hibridizacija
Hybridization is an internal linear combination of atomic orbitals, in which the wave functions of the atomic orbitals are added together to generate new hybrid wave functions. The new orbitals which are formed are hybrids of the originals and have properties (shape, size and energy) that are somewhere in between.
Gaussian system of units → Gaussov sustav jedinica
Gaussian system of units is a hybrid system used in electromagnetic theory, which combines features of both the electrostatic cgs subsystem (esu) and electromagnetic cgs subsystem (emu). With three base units, it uses em units in magnetism and es units in electrostatics. This involves using the constant c (the velocity of light in vacuum) to interrelate these sets of units.
linear molecular geometry → linearna geometrija molekule
Linear molecule is a molecule in which atoms are deployed in a straight line (under 180° angle). Molecules with an linear electron pair geometries have sp hybridization at the central atom. An example of linear electron pair and molecular geometry are carbon dioxide (O=C=O) and beryllium hydride BeH2.
valence bond theory → teorija valentne veze
Valence bond theory is a theory that explains the shapes of molecules in terms of overlaps between half-filled atomic orbitals, or half filled hybridised orbitals.
molecular shape → oblik molekule
Molecular shape is the three dimensional arrangement of atoms in space around a central atom. The molecular formula of a substance does not give an indication of its shape. For example, CO2 is a linear molecule, but SO2 is angular.
The three-dimensional shapes of many small molecules can be predicted by applying the valence shell electron pair repulsion theory (VSEPR). When atoms combine to form molecules, pairs of valence electrons arrange themselves as far from each other as possible. Another way to characterize molecular shape is in terms of hybrid orbitals.
octahedral molecular geometry → oktaedarska geometrija molekule
Octahedral molecular geometry (square bipyramidal shape) describes the shape of compounds where six atoms or ligands are symmetrically arranged around a central atom. The sulfur hexafluoride (SF6), with six bonding pairs, is predicted and found to be a regular octahedron. Four of the attachments are positioned in a square plane with 90° bond angles. The remaining two attachments are positioned perpendicular (90°) to the square plane at opposite ends of the central atom. Molecules with an octahedral electron pair geometries have sp3d2 (or d2sp3) hybridization at the central atom.
Citing this page:
Generalic, Eni. "Toyota harrier hybrid." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table