graphite → grafit
Graphite is an allotrope of carbon. The atoms are arranged in layers as a series of flat, hexagonal rings. Graphite is a good conductor of heat and electricity. The layers cleave easily, making graphite useful as a solid lubricant. A process to make pure synthetic graphite was invented by the American chemist Edward Goodrich Acheson (1856–1931). The process consists of heating a mixture of clay (aluminum silicate) and powdered coke (carbon) in an iron bowl. The reaction involves the production of silicon carbide, which loses silicon at 4150 °C to leave graphite.
halocarbon → halogenirani ugljikovodik
Halocarbon is a compound containing no elements other than carbon, one or more halogens, and sometimes hydrogen. The simplest are compounds such as tetrachloromethane (CCl4), tetrabromomethane (CBr4), etc. The lower members of the various homologous series are used as refrigerants, propellant gases, fireextinguishing agents, and blowing agents for urethane foams. When polymerized, they yield plastics characterized by extreme chemical resistance, high electrical resistivity, and good heat resistance.
hardness → tvrdoća
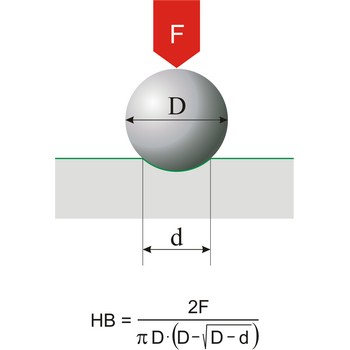
Hardness is the resistance of a material to deformation of an indenter of specific size and shape under a known load. This definition applies to all types of hardness scales except Mohs scale, which is a based on the concept of scratch hardness and is used chiefly for minerals. The most generally used hardness scales are Brinell (for cast iron), Rockwell (for sheet metal and heat-treated steel), Knoop (for metals).
racemisation → racemizacija
Racemisation is a conversion, by heat or by chemical reaction, of an optically active compound into an optically inactive form which half of the optically active substance becomes its miror image (enantiomer).
radiation → radijacija
The process of heat transfer which occurs through empty space and can also occur in matter, in the form of electro-magnetic (EM) waves, is called radiation or radiant heat. Whenever EM radiation is emitted and then absorbed, heat is transferred. This principle is used in microwave ovens, laser cutting, and RF hair removal.
Rankine cycle → Rankineov ciklus
Rankine cycle is a thermodynamic cycle which can be used to calculate the ideal performance of a heat engine that uses a condensable vapour as the working fluid.
Hesse’s law → Hessov zakon
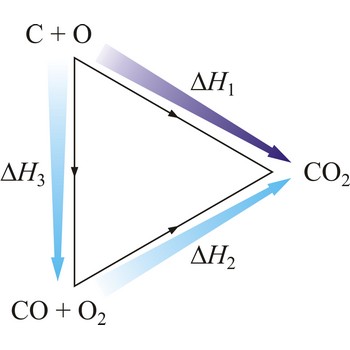
Hesse’s law says that reaction heat of some chemical change does not depend on the way in which the reaction is conducted, but only on starting and ending system state. Hesse’s law is also known as the law of constant heat summation. Hesse’s law is also known as the law of constant heat summation. The law was first put forward in 1840 by the Swiss-born Russian chemist Germain Henri Hess (1802-1850).
Hesse’s law can be used to obtain thermodynamic data that cannot be measured directly. For example, it is very difficult to control the oxidation of graphite to give pure CO. However, enthalpy for the oxidation of graphite to CO2 can easily be measured. So can the enthalpy of oxidation of CO to CO2. The application of Hess’s law enables us to estimate the enthalpy of formation of CO.
| C(s) + O2(g) →← CO2(g) | ΔrH1 = -393 kJ mol-1 |
| CO(g) + 1/2O2(g) →← CO2(g) | ΔrH2 = -283 kJ mol-1 |
| C(s) + 1/2O2(g) →← CO(g) | ΔrH3 = -110 kJ mol-1 |
The equation shows the standard enthalpy of formation of CO to be -110 kJ/mol.
hydration → hidratacija
Hydration is addition of water or the elements of water (i.e. H and OH) to a molecular entity. The term is also used in a more restricted sense for the process:
specific quantity → specifična veličina
Specific quantity is often convenient to express an extensive quantity (e.g., volume, enthalpy, heat capacity, etc.) as the actual value divided by mass. The resulting quantity is called specific volume, specific enthalpy, etc.
Citing this page:
Generalic, Eni. "Toplina izgaranja." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table