Lewis, Gilbert N. → Lewis, Gilbert N.
Gilbert Newton Lewis (1875-1946) is an American chemist whose theory of the electron pair fostered understanding of the covalent bond and extended the concept of acids and bases.
electrical double layer → električni dvosloj
Electrical double layer is the structure of charge accumulation and charge separation that always occurs at the interface when an electrode is immersed into an electrolyte solution. The excess charge on the electrode surface is compensated by an accumulation of excess ions of the opposite charge in the solution. The amount of charge is a function of the electrode potential. This structure behaves essentially as a capacitor. There are several theoretical models that describe the structure of the double layer. The three most commonly used ones are the Helmholtz model, the Gouy-Chapman model, and the Gouy-Chapman-Stern model.
enzyme → enzim
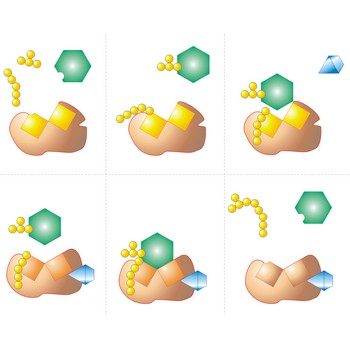
Enzyme is a protein that acts as a catalyst in biochemical reactions. Each enzyme is specific to a particular reaction or a group of similar reactions. Many require the association of certain nonprotein cofactors in order to function. The molecule undergoing a reaction (the substrate) binds to a specific active site on the enzyme molecule to form a short-lived intermediate: this greatly increases (by a factor of up to 1020) the rate at which the reaction proceeds to form the product.
Fischer-Tropsch process → Fischer-Tropschov postupak
Fischer-Tropsch process is an industrial method of making hydrocarbon fuels from carbon monoxide and hydrogen. The process was introduced in 1933. and used by Germany in World War II. to produce motor fuel. Hydrogen and carbon monoxide are mixed in the ratio 2:1 (water gas was used with added hydrogen) and passed at 200 °C over a nickel or cobalt catalyst. The resulting hydrocarbon mixture can be separated into a higher-boiling fraction for Diesel engines and a lower-boiling petrol fraction. The petrol fraction contains a high proportion of straight-chain hydrocarbons and has to be reformed for use in motor fuel. Alcohols, aldehydes, and ketones are also present. The process is also used in the manufacture of SNG from coal. It is named after the German chemist Franz Fischer (1852-1932) and the Czech Hans Tropsch (1839-1935).
Haber process → Haberov proces
Haber process is an industrial process for producing ammonia by reaction of nitrogen with hydrogen:
The reaction is reversible and exothermic, so that a high yield of ammonia is favoured by low temperature. However, the rate of reaction would be too slow for equilibrium to be reached at normal temperatures, so an optimum temperature of about 450 °C is used, with a catalyst of iron containing potassium aluminium oxide promoters. The higher the pressure the greater the yield, although there are technical difficulties in using very high pressures. A pressure of about 250 atmospheres is commonly employed. The removal of ammonia from the batch as soon as it is formed ensures that an equilibrium favouring product formation is maintained. The nitrogen is obtained from air. Formerly, the hydrogen was from water gas and the water-gas shift reaction (the Bosch process) but now the raw material (called synthesis gas) is obtained by steam reforming natural gas.
The process is of immense importance for the fixation of nitrogen for fertilisers and explosives. It was developed in 1908 by German chemist Fritz Haber (1868-1934) and was developed for industrial use by Carl Bosch (1874-1940), hence the alternative name Haber-Bosch process.
haematite → hematit
Haematite is a mineral of iron(III) oxide Fe2O3. It is the most important ore of iron and usually occurs in two main forms: as a massive red kidney-shaped ore and as grey to black metallic crystals known as specular iron ore. Haematite is the major red colouring agent in rocks; the largest deposits are of sedimentary origin. In industry haematite is also used as a polishing agent (jeweller’s rouge) and in paints.
hydrolysis → hidroliza
Hydrolysis is a chemical reaction in which water reacts with another substance to form two or more new substances. This involves ionisation of the water molecule, as well as splitting of the compound hydrolysed, e.g.
Examples are conversion of starch to glucose by water in the presence of suitable catalysts and a reaction of the ions of a dissolved salt to form various products, such as acids, complex ions, etc.
Kjeldahl’s method → Kjeldahlov postupak
Kjeldahl’s method is an analytical method for determination of nitrogen in certain organic compounds. The method was developed by the Danish chemist Johan Kjeldahl (1849-1900).
It involves addition of a small amount of anhydrous potassium sulphate to the test compound, followed by heating the mixture with concentrated sulphuric acid, often with a catalyst such as copper sulphate. As a result ammonia is formed. After alkalyzing the mixture with sodium hydroxyde, the ammonia is separated by distillation, collected in standard acid, and the nitrogen determined by back-titration.
- Kjeldahl flask for decomposition (500 ml – macro or 100 ml - micro)
- funnel for alkaline solution
- Wagner tube (drop catcher)
- condenser
- absorption flask with known volume of standard acid
Citing this page:
Generalic, Eni. "Teorije katalize." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table