lateral chain → postranični lanac
Lateral chain is a shorter chain of hydrocarbons which is connected to the main chain of hydrocarbon.
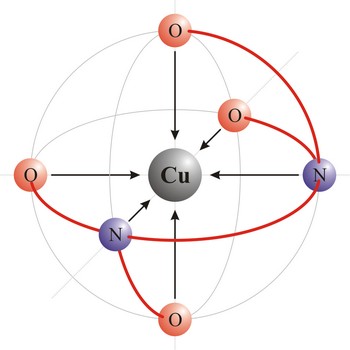
ligand → ligand
Ligand is an ion (F-, Cl-, Br-, I-, S2-, CN-, NCS-, OH-, NH2-) or molecule (NH3, H2O, NO, CO) that donates a pair of electrons to a metal atom or ion in forming a coordination complex. The main way of classifying ligands is by the number of points at which they are attached to, or bound to, the metal center. This is the denticity. Ligands with one potential donor atom are monodentate. Polydentate ligand is a ligand that is attached to a central metal ion by bonds from two or more donor atoms. Ligands with more than one potential donor atom are known as ambidentate, such as the thiocyanate ion, NCS-, which can bind to the metal center with either the nitrogen or sulphur atoms. Chelating ligands are those polydentate ligands which can form a ring including the metal atom.
macromolecule → makromolekule
Macromolecule is a molecule of high relative molecular mass (molecular weight), the structure of which essentially comprises the multiple repetitions of units derived, actually or conceptually, from molecules of low relative molecular mass. The types of macromolecules are natural and synthetic polymers, carbohydrates, lipids, proteins etc. Cellulose is a polysaccharide that is made up of hundreds, even thousands of glucose molecules strung together.
mutarotation → mutarotacija
Mutarotation is the change in optical rotation accompanying epimerization. In carbohydrate chemistry this term usually refers to epimerization at the hemiacetal carbon atom. In general α- and β-form are stable solids, but in solution they rapidly equilibrate. For example, D-glucose exists in an equilibrium mixture of 36 % α-D-glucopyranose and 64 % β-D-glucopyranose, with only a tiny fraction in the open-chain form. The equilibration occurs via the ring opening of the cyclic sugar at the anomeric center with the acyclic form as the intermediate. Mutarotation was discovered by French chemist Augustin-Pierre Dubrunfaut (1797-1881) in 1846.
monosaccharide → monosaharid
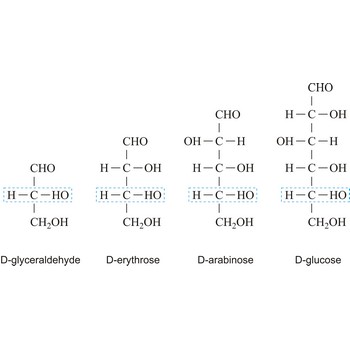
Monosaccharides are carbohydrates, with the general formula Cn(H2O)n, that cannot be decomposed to a simpler carbohydrates by hydrolysis.
Depending on whether the molecule contains an aldehyde group (-CHO) or a ketone group (-CO-) monosaccharide can be a polyhydroxy aldehyde (aldose) or a polyhydroxy ketone (ketose). These aldehyde and ketone groups confer reduction properties on monosaccharides. They are also classified according to the number of carbon atoms they contain: trioses have three carbon atoms, tetroses four, pentoses five, hexoses six, heptoses seven, etc. These two systems of classification are often combined. For example, a six-carbon polyhydroxy aldehyde such as D-glucose is an aldohexose, whereas a six-carbon polyhydroxy ketone such as D-fructose is a ketohexose.
The notations D and L are used to describe the configurations of carbohydrates. In Fischer projections of monosaccharides, the carbonyl group is always placed on top (in the case of aldoses) or as close to the top as possible (in the case of ketoses). If the OH group attached to the bottom-most asymmetric carbon (the carbon that is second from the bottom) is on the right, then the compound is a D-sugar. If the OH group is on the left, then the compound is an L-sugar. Almost all sugars found in nature are D-sugars.
Monosaccharides can exist as either straight-chain or ring-shaped molecules. During the conversion from straight-chain form to cyclic form, the carbon atom containing the carbonyl oxygen, called the anomeric carbon, becomes a chiral center with two possible configurations (anomers), α and β. When the stereochemistry of the first carbon matches the stereochemistry of the last stereogenic center the sugar is the α-anomer when they are opposite the sugar is the β-anomer.
nucleotide → nukleotid
Nucleotides are the components that made up nucleic acids. They have three major components: the first component is a nitrogenous base, which is derivative of one of two parent compounds, pyrimidine or purine; the second is a pentose, or five carbon sugar group; the third is a unit of phosphate. Each group of three nucleotides in a gene is known as a codon. Whenever the phosphate group is not present, a nucleotide becomes a nucleoside.
omega-3 fatty acids → omega-3 masne kiseline
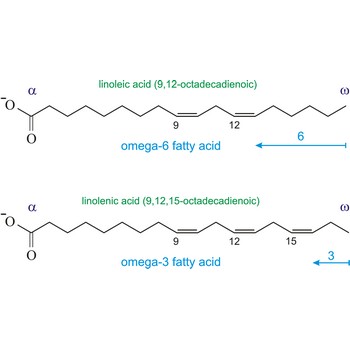
Omega-3 fatty acids are polyunsaturated fatty acids, meaning they contain more than one double bond. The name omega-3 indicates that the first double bond occurs on the third carbon atom (n-3) from the methyl (-CH3) end of the molecule (omega position). The three main omega-3 fatty acids are alpha-linolenic acid (ALA, 18:3n-3), eicosapentaenoic acid (EPA, 20:5n-3), and docosahexaenoic acid (DHA, 22:6n-3). ALA comes from plants. EPA and DHA come from fish.
Similarly, the first double bond in omega-6 fatty acids is located between the sixth and seventh carbon atom (n-6) from the methyl end of the fatty acid (omega end).
Ostwald’s process → Ostwaldov proces
Ostwald’s process is a process by which the nitric acid can be obtained in three degrees. In the first stage ammonia and oxygen react (with platinum-rhodium as a catalyst), whereby the nitrogen monoxide and water emerge
In the second stage nitrogen monoxide reacts with oxygen whereby nitrogen dioxide emerges
and in the third stage nitrogen dioxide dissolves in water, in the presence of air, giving the nitric acid
Citing this page:
Generalic, Eni. "Superkritični ugljikov dioksid." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table