plastic sulphur → plastični sumpor
Plastic sulphur is the shape of sulphur which emerges when hot, liquid sulphur pours out in the water. It can be stretched in long fibres is unstable unstable. It easily solidifies.
sulfur → sumpor
Sulfur has been known since ancient times. The origin of the name comes from the Sanskrit word sulvere meaning sulphur; also from the Latin word sulphurium meaning sulphur. It is pale yellow, odourless, brittle solid, which is insoluble in water but soluble in carbon disulfide. Sulfur is found in pure form and in ores like cinnabar, galena, sphalerite and stibnite. Pure form is obtained from underground deposits by the Frasch process. Used in matches, gunpowder, medicines, rubber and pesticides, dyes and insecticides. Also for making sulfuric acid (H2SO4).
acid → kiselina
Acid is a type of compound that contains hydrogen and dissociates in water to produce positive hydrogen ions. The reaction for an acid HA is commonly written:
In fact, the hydrogen ion (the proton) is solvated, and the complete reaction is:
This definition of acids comes from the Arrhenius theory. Such acids tend to be corrosive substances with a sharp taste, which turn litmus red and produce colour changes with other indicators. They are referred to as protonic acids and are classified into strong acids, which are almost completely dissociated in water, (e.g. sulphuric acid and hydrochloric acid), and weak acids, which are only partially dissociated (e.g. acetic acid and hydrogen sulphide). The strength of an acid depends on the extent to which it dissociates, and is measured by its dissociation constant.
In the Lowry-Brønsted theory of acids and bases (1923), the definition was extended to one in which an acid is a proton donor (a Brønsted acid), and a base is a proton acceptor (a Brønsted base). An important feature of the Lowry-Brønsted concept is that when an acid gives up a proton, a conjugate base is formed that is capable of accepting a proton.
Similarly, every base produces its conjugate acid as a result of accepting a proton.
For example, acetate ion is the conjugate base of acetic acid, and ammonium ion is the conjugate acid of ammonia.
As the acid of a conjugate acid/base pair becomes weaker, its conjugate base becomes stronger and vice versa.
A further extension of the idea of acids and bases was made in the Lewis theory. In this, a G. N. Lewis acid is a compound or atom that can accept a pair of electrons and a Lewis base is one that can donate an electron pair. This definition encompasses "traditional" acid-base reactions, but it also includes reactions that do not involve ions, e.g.
in which NH3 is the base (donor) and BCl3 the acid (acceptor).
acid rain → kisela kiša
Acid rain is rainwater that shows acid reaction because of nitrogen and sulphur oxides absorption. It is generated mainly by industrial pollutions.
battery acid → baterijska kiselina
Battery acid is a solution of approximately 6 mol L-1 sulphuric acid used in the lead storage battery
blanching → blanširanje
1. Blanching is a heat treatment of foodstuffs to partially or completely inactivate the naturally occurring enzymes prior to freezing.
2. Blanching is a washing process for coins cleaning. The black surface layer of cupric oxide is removing by dipping the coins in hot dilute sulphuric acid (w(H2SO4) = 10 %).
Californian soup → kalifornijska juha
Californian soup is a fungicide and insecticide chemical used for protection of plants, manufactured by boiling sulphur with slaked lime.
catalytic hydrogenation → katalitičko hidrogeniranje
Catalytic hydrogenation is the infusing of unsaturated or impure hydrocarbons with hydrogen gas at controlled temperatures and pressures and in the presence of a catalyst for the purpose of obtaining saturated hydrocarbons and/or removing various impurities such as sulphur and nitrogen.
anhydrous → anhidrid
Anhydrous (without water) is an applied to minerals which do not contain water of crystallization or water of chemical combination. For example, strongly heated copper (II) sulphate pent hydrate (CuSO4•5H2O) produces anhydrous copper (II) sulphate (CuSO4). Less stable and more dangerous to use than hydrated.
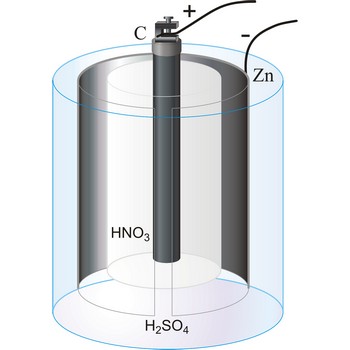
Bunsen’s cell → Bunsenov članak
Bunsen’s cell is a primary cell devised by Robert W. Bunsen consisting of a zinc cathode immersed in dilute sulphuric acid and carbon anode immersed in concentrated nitric acid. The electrolytes are separated by a porous pot. The cell gives an e.m.f. of about 1.9 V.
Citing this page:
Generalic, Eni. "Sumpor." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table