equilibrium constant → konstanta ravnoteže
The equilibrium constant (K) was originally introduced in 1863 by Norwegian chemists C.M. Guldberg and P. Waage using the law of mass action. For a reversible chemical reaction represented by the equation
chemical equilibrium occurs when the rate of the forward reaction equals the rate of the back reaction, so that the concentrations of products and reactants reach steady-state values.
The equilibrium constant is the ratio of chemical activities of the species A, B, C, and D at equilibrium.
To a certain approximation, the activities can be replaced by concentrations.
For gas reactions, partial pressures are used rather than concentrations
The units of Kp and Kc depend on the numbers of molecules appearing in the stoichiometric equation (a, b, c, and d).
The value equilibrium constant depends on the temperature. If the forward reaction is exothermic, the equilibrium constant decreases as the temperature rises. The equilibrium constant shows the position of equilibrium. A low value of K indicates that [C] and [D] are small compared to [A] and [B]; i.e. that the back reaction predominates.
The equilibrium constant is related to ΔrG°, the standard Gibbs free energy change in the reaction, by
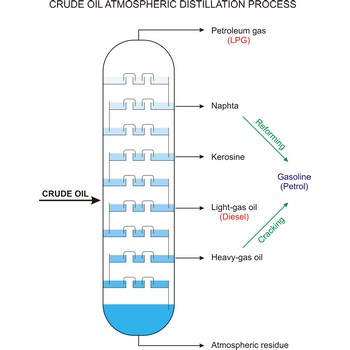
fractional distillation → frakcijska destilacija
Fractional distillation is a procedure in which liquids of close boiling points are separated. It is conducted in fraction or rectification columns in a way that vapour phase created by distillation is condensed and the condensate thus obtained is redistilled. The procedure is repeated several times. Vapour phase always contains more volatile component than the liquid phase, at top of the column vapour of clean volatile component gets out and at the bottom of the column liquid of nonvolatile component.
freon → freon
Freon (chlorofluorocarbon, CFC) a type of compound in which some or all of the hydrogen atoms of hydrocarbon (usually an alkane) have been replaced by chlorine and fluorine atoms. Most CFC are chemically uncreative and are stable at high temperatures. They are used as aerosol propellants, refrigerants, and solvents, and in the manufacture of rigid packaging foam. CFC because of their chemical inertness, can diffuse unchanged into the upper atmosphere. Here, photochemical reactions cause them to break down and react with ozone. For his reason, their use has been discouraged.
fugacity → fugacitet
Fugacity (f) is a thermodynamic function used in place of partial pressure in reactions involving real gases and mixtures. For a component of a mixture, it is defined by
where μ is the chemical potential.
The fugacity of a gas is equal to the pressure if the gas is ideal. The fugacity of a liquid or solid is the fugacity of the vapour with which it is in equilibrium. The ratio of the fugacity to the fugacity in some standard state is the activity.
global warming → globalno zatopljenje
Global warming or greenhouse effect is an effect occurring in the atmosphere because of the presence of certain gases (greenhouse gases) that absorb infrared radiation. Light and ultraviolet radiation from the sun is able to penetrate the atmosphere and warm the Earth’s surface. This energy is re-radiated as infrared radiation which because of its longer wavelength, is absorbed by such substances as carbon dioxide. The overall effect is that the average temperature of the Earth and its atmosphere is increasing (so-called global Warming). The effect is similar to that occurring in a greenhouse, where light and long-wavelength ultraviolet radiation can pass through the glass into greenhouse but the infrared radiation is absorbed by the glass and part of it is re-radiated into the greenhouse.
The greenhouse effect is seen as a major environmental hazard. Average increases in temperature could change weather patterns and agricultural output. It might also lead to melting of the polar ice caps and a corresponding rise in sea level. Carbon dioxide, from fossil-fuel power stations and car exhausts, is the main greenhouse gas. Other contributory pollutants are nitrogen oxides, ozone, methane, and chloroflourocarbons.
Haber process → Haberov proces
Haber process is an industrial process for producing ammonia by reaction of nitrogen with hydrogen:
The reaction is reversible and exothermic, so that a high yield of ammonia is favoured by low temperature. However, the rate of reaction would be too slow for equilibrium to be reached at normal temperatures, so an optimum temperature of about 450 °C is used, with a catalyst of iron containing potassium aluminium oxide promoters. The higher the pressure the greater the yield, although there are technical difficulties in using very high pressures. A pressure of about 250 atmospheres is commonly employed. The removal of ammonia from the batch as soon as it is formed ensures that an equilibrium favouring product formation is maintained. The nitrogen is obtained from air. Formerly, the hydrogen was from water gas and the water-gas shift reaction (the Bosch process) but now the raw material (called synthesis gas) is obtained by steam reforming natural gas.
The process is of immense importance for the fixation of nitrogen for fertilisers and explosives. It was developed in 1908 by German chemist Fritz Haber (1868-1934) and was developed for industrial use by Carl Bosch (1874-1940), hence the alternative name Haber-Bosch process.
hydrogen → vodik
Hydrogen was discovered by Sir Henry Cavendish (England) in 1766. The origin of the name comes from the Greek words hydro and genes meaning water and generate. It is colourless, odourless gas, burns and forms explosive mixtures in air. Reacts violently with oxidants. Hydrogen is the most abundant element in the universe. Commercial quantities of hydrogen are produced by reacting superheated steam with methane or carbon. In lab work from reaction of metals with acid solutions or electrolysis. Most hydrogen is used in the production of ammonia and in metal refining. Also used as fuel in rockets. Its two heavier isotopes (deuterium and tritium) used respectively for nuclear fusion.
hydrosphere → hidrosfera
Hydrosphere (from the Greek for water sphere) is a discontinuous layer of water on, under, and over the Earth's surface. It includes all liquid and frozen surface waters, groundwater held in soil and rock, and atmospheric water vapour. Water continuously circulates between these reservoirs in what is called the hydrologic cycle, which is driven by energy from the Sun.
| Reservoir | V / 106 km3 | w / % |
|---|---|---|
| oceans | 1 370.0 | 97.25 |
| ice caps and glaciers | 29.0 | 2.05 |
| groundwater | 9.5 | 0.68 |
| lakes, rivers | 0.127 | 0.01 |
| soil moisture | 0.065 | 0.005 |
| atmosphere (as liquid equivalent of water vapour) | 0.013 | 0.001 |
| biosphere | 0.0006 | 0.00004 |
| TOTAL | 1 408.7 | 100 |
Kirchoff, Gustav → Kirchoff, Gustav
Gustav Kirchoff (1824-1887) was a German physicist who, with the chemist Robert Bunsen (1811-1899), laid the foundations of spectral analysis. He realized that the Fraunhofer lines in the Sun's spectrum were due to light from the photosphere being absorbed at those specific wavelengths by elements in the solar atmosphere. He also found that incandescent solids, liquids, and compressed gases emit a continuous spectrum. Use of the Bunsen burner in conjunction with a glass prism led to the development of the spectroscope in collaboration with the Bunsen and to the spectroscopic discovery of the elements rubidium (1860) and cesium (1861).
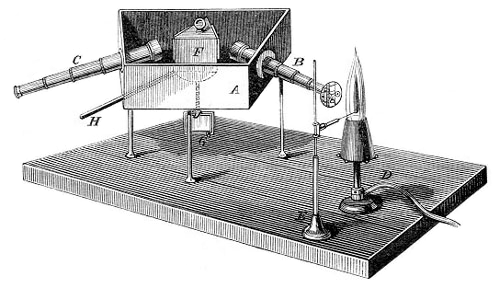
Kjeldahl’s method → Kjeldahlov postupak
Kjeldahl’s method is an analytical method for determination of nitrogen in certain organic compounds. The method was developed by the Danish chemist Johan Kjeldahl (1849-1900).
It involves addition of a small amount of anhydrous potassium sulphate to the test compound, followed by heating the mixture with concentrated sulphuric acid, often with a catalyst such as copper sulphate. As a result ammonia is formed. After alkalyzing the mixture with sodium hydroxyde, the ammonia is separated by distillation, collected in standard acid, and the nitrogen determined by back-titration.
- Kjeldahl flask for decomposition (500 ml – macro or 100 ml - micro)
- funnel for alkaline solution
- Wagner tube (drop catcher)
- condenser
- absorption flask with known volume of standard acid
Citing this page:
Generalic, Eni. "Standardna atmosfera." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table