electron spin → elektronski spin
Electron spin (s) is the quantum number, equal to 1/2, that specifies the intrinsic angular momentum of the electron.
spin pair → spinski par
Spin pair (↑↓) is two electrons with opposite spins, usually occupying the same orbital.
spin quantum number → spinski kvantni broj
Spin quantum number (ms) is the quantum number for the electron having values +1⁄2 and -1⁄2. Serves to differentiate between two electrons in the same orbital.
spin → spin
Spin is the intrinsic angular momentum of an elementary particle, or system of particles such as nucleus, that is also responsible for the magnetic moment; or, a particle or nucleus possessing such a spin. The spins of nuclei have characteristic fixed values. Pairs of neutrons and protons align to cancel out their spins, so that nuclei with an odd number of neutrons and/or protons will have a net non-zero rotational component characterized by a non-zero quantum nuclear spin number.
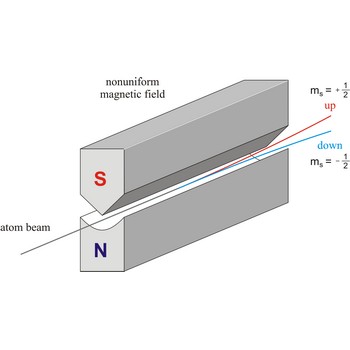
Stern-Gerlach experiment: a beam of silver atoms is split into two beams when it traverses a nonuniform magnetic field. Atoms with spin quantum number ms=+1/2 follow one trajectory, and those with ms=+1/2 follow another.
white spirit → bijeli špirit
White spirit (mineral spirits, petroleum spirits) is a paraffin-derived clear, transparent liquid which is a common organic solvent used in painting and decorating.
Bohr magneton → Bohrov magneton
Bohr magneton (μB) is the atomic unit of magnetic moment, defined as
where h is Planck’s constant, me the electron mass, and e the elementary charge. It is the moment associated with a single electron spin.
cobalt → kobalt
Cobalt was discovered by Georg Brandt (Germany) in 1735. The origin of the name comes from the German word kobald meaning goblin or evil spirit. It is hard, ductile, lustrous bluish-grey metal. Surfaces stable in air. Reacts over time with dilute acids. It has remarkable magnetic properties. Cobalt occurs in compounds with arsenic and sulfur as in cobaltine (CoAsS) and linneite (Co3S4). Pure cobalt is obtained as a by-product of refining nickel, copper and iron. Used in many hard alloys; for magnets, ceramics and special glasses. Radioactive cobalt-60 is used in cancer therapy.
diamagnetic → dijamagnetik
Diamagnetic materials are very weakly repelled by magnetic fields. The atoms or molecules of diamagnetic materials contain no unpaired spins.
electron pair → elektronski par
Electron pair is two electrons within one orbital with opposite spins responsible for a chemical bond.
Citing this page:
Generalic, Eni. "Spin." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table