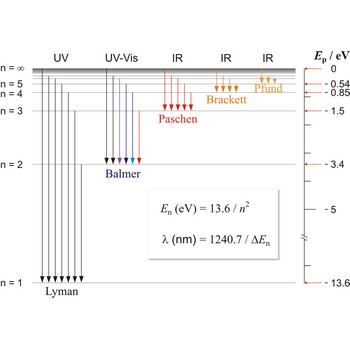
Balmer series → Balmerova serija
Balmer series, Balmer lines is a series of lines in the emission spectrum of hydrogen that involve transitions to the n=2 state from states with n>2.
barium → barij
Barium was discovered by Sir Humphry Davy (England) in 1808. The origin of the name comes from the Greek word barys meaning heavy. It is soft, slightly malleable, silvery-white metal. Attacked by air and water. Soluble compounds toxic by ingestion. Barium is found in barytine (BaSO4) and witherite (BaCO3), never found in pure form due to its reactivity. Must be stored under kerosene to remain pure. Barite, or barium sulfate (BaSO4), when ground is used as a filter for rubber, plastics and resins. It is insoluble in water and so is used in X-rays of the digestive system. Barium nitrate, Ba(NO3)2, burns brilliant green and is used in fireworks.
benzene → benzen
Benzene is a colourless liquid hydrocarbon, C6H6, b.p. 80 °C. It is now made from petroleum by catalytic reforming (formerly obtained from coal tar). Benzene is the archetypal aromatic compound. It has an unsaturated molecule, yet will not readily undergo addition reactions. On the other hand, it does undergo substitution reactions in which hydrogen atoms are replaced by other atoms or groups.
In 1865, Friedrich August Kekulé purposed the benzene molecule structure as a hexagonal ring which consists of six carbon atoms with alternate carbon-carbon single and carbon-carbon double bond. But such a structure should be highly reactive, and so didn't account for the unreactive nature of benzene. We now know that the best representation for the structure of benzene is indeed, hexagonal, with each C-C bond distance being identical and intermediate between those for a single and double bond. The π-orbitals from each neighbouring carbon atom overlap to form a delocalised molecular orbital which extends around the ring, giving added stability and with it, decreased reactivity. That is the reason the structural formula of benzene represents as a hexagon with a circle in the center which represents the delocalized electrons.
contaminant → kontaminat
Contaminants are any physical, chemical, biological, or radiological substance or matter in water that may be harmful to human health or which degrade the palatability of water.
cyanide process → cijanidni postupak
Cyanide process is a method for separating a metal from an ore. Crushed ore is treated with cyanide ion to produce a soluble metal cyanide complex. The complex is washed out of the ore and reduced to metallic form using an active metal (usually zinc).
beryllium → berilij
Beryllium was discovered by Friedrich Wöhler (Germany) and independently by A. B. Bussy (France) in 1828. The origin of the name comes from the Greek word beryllos meaning mineral beryl; also called glucinium from the Greek word glykys meaning sweet. It is steel-grey metal. It resists attack by concentrated nitric acid, has excellent thermal conductivity and is nonmagnetic. At ordinary temperatures, it resists oxidation in air. Beryllium and its salts are toxic and should be handled with the greatest of care. Beryllium is found mostly in minerals like beryl [AlBe3(Si6O18)] and chrysoberyl (Al2BeO4). Pure beryllium is obtained by chemically reducing beryl mineral. Also by electrolysis of beryllium chloride. Its ability to absorb large amounts of heat makes it useful in spacecraft, missiles, aircraft, etc. Emeralds are beryl crystals with chromium traces giving them their green colour.
bismuth → bizmut
Bismuth was discovered by Claude Geoffroy (France) in 1753. The origin of the name comes from the German words Weisse Masse meaning white mass; now spelled wismut and bisemutum. It is hard, brittle, steel-grey metal with a pink tint. Stable in oxygen and water. Dissolves in concentrated nitric acid. Bismuth can be found free in nature and in minerals like bismuthine (Bi2S3) and in bismuth ochre (Bi2O3) Main use is in pharmaceuticals and low melting point alloys used as fuses.
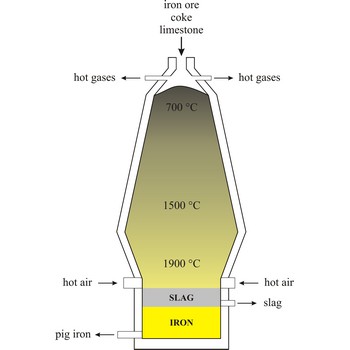
blast furnace → visoka peć
Blast furnace is a furnace for smelting of iron from iron oxide ores (hematite, Fe2O3 or magnetite, Fe3O4). Coke, limestone and iron ore are poured in the top, which would normally burn only on the surface. The hot air blast to the furnace burns the coke and maintains the very high temperatures that are needed to reduce the ore to iron. The reaction between air and the fuel generates carbon monoxide. This gas reduces the iron(III) oxide in the ore to iron.
Because the furnace temperature is in the region of 1500 °C, the metal is produced in a molten state and this runs down to the base of the furnace.
The production of iron in a blast furnace is a continuous process. The furnace is heated constantly and is re-charged with raw materials from the top while it is being tapped from the bottom. Iron making in the furnace usually continues for about ten years before the furnace linings have to be renewed.
flash point → temperatura zapaljenja
Flash point is the lowest temperature at which a liquid or volatile solid gives off vapour sufficient to form an ignitable mixture with the air near the surface of the liquid or within the test vessel (NFPA).
Citing this page:
Generalic, Eni. "Spektar X-zraka." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table