fluorine → fluor
Fluorine was discovered by Henri Moissan (France) in 1886. The origin of the name comes from the Latin word fluere meaning to flow. It is pale yellow to greenish gas, with an irritating pungent odour. Extremely reactive, flammable gas. Reacts violently with many materials. Toxic by inhalation or ingestion. Does not occur uncombined in nature. Fluorine is found in the minerals fluorite (CaF2) and cryolite (Na3AlF6). Electrolysis of hydrofluoric acid (HF) or potassium acid fluoride (KHF2) is the only practical method of commercial production. Used in refrigerants and other fluorocarbons. Also in toothpaste as sodium fluoride (NaF).
heavy water → teška voda
Water molecules are composed of two hydrogen atoms and one oxygen atom (H2O). If the hydrogen atoms of a water molecule are replaced by deuterium atoms, the result is heavy water (D2O). Deuterium differs from hydrogen by having one neutron in the nucleus of the atom. There is approx. one part in 5000 D2O in normal water and it can be concentrated by electrolysis. Heavy water has a higher boiling point (101.4 °C) and melts at 3.6 °C. Heavy water is 20/18=1.11 times heavier than ordinary water.
melting point → talište
Melting point is the temperature at which a solid becomes a liquid at normal atmospheric pressure.
A more specific definition of melting point (or freezing point) is the temperature at which the solid and liquid phases of a substance are in equilibrium at a specified pressure (normally taken to be atmospheric unless stated otherwise). A pure substance under standard condition of pressure has a single reproducible melting point. The terms melting point and freezing point are often used interchangeably, depending on whether the substance is being heated or cooled.
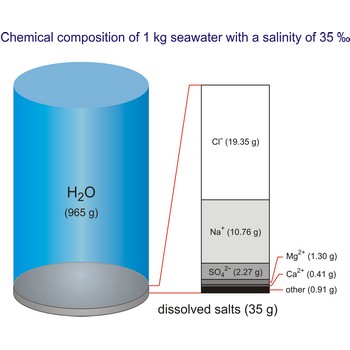
seawater → more
Seawater is a complex mixture of 96.5 % water, 3.5 % salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates, and a few atmospheric gases. The world's oceans cover nearly 71 % (361 840 000 km2) of the Earth's surface (510 100 000 km2), with an average depth of 3 682.2 m.
The density of seawater is higher than that of fresh water because of its higher salinity. Seawater's freezing point is lower than that of pure water and its boiling point is higher. The average salinity of the ocean is 35 ‰, which means that for every kilograms of water, there are 35 g of salt. The relative abundance of the major salts in seawater are constant regardless of the ocean. Only six elements and compounds comprise about 99 % of sea salts: chlorine (Cl-), sodium (Na+), sulfur (SO42-), magnesium (Mg2+), calcium (Ca2+), and potassium (K+).
Citing this page:
Generalic, Eni. "Snižavanje ledišta." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table