Results 1–9 of 9 for sapun
detergent → deterdžent
Detergent is a substance added to water to improve its cleaning properties. Although water is a powerful solvent for many compounds, it will not dissolve grease and natural oils. Detergents are compounds that cause such nonpolar substances to go into solution in water. Soap is the original example, owing its action to the presence of ions formed from long-chain fatty acids ion (e.g. stearat ion, CH3(CH2)16COO-).
fat → mast
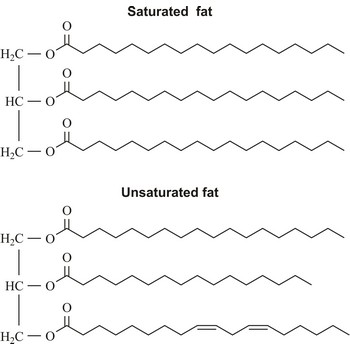
Fats are esters of glycerol and long chain carboxylic acids. Fats occur widely in plants and animals as a means of storing food energy, having twice the calorific value of carbohydrates. Fats derived from plants and fish generally have a greater proportion of unsaturated fatty acids than those from mammals. Fats may be either solid or liquid at room temperature, depending on their structure and composition. Unsaturated fats are liquid at room temperature.
Plant oils may be hardened by the addition of hydrogen atoms, converting double bonds to single bonds. This process is known as hydrogenation. Hydrogenated vegetable oils are often present in margarine and other processed foods.
Alkali hydrolysis of fat with sodium hydroxide it gives glycerol and soap (i.e. a mixture of the sodium salts of the fatty acids).
foam → pjena
Foams are dispersions of gases in liquids or solids. The gas globule may be of any size, from colloidal to macroscopic, as in soap bubbles. Bakers’ bread and sponge rubber are examples of solid foams. Typical liquid foams are those used in fire-fighting, shaving creams, etc. Foams made by mechanical incorporation of air are widely used in the food industry (e.g. whipped cream, egg white, ice cream, etc.). Foams can be stabilized by surfactants.
micelle → micele
Micelle is an electrically charged colloidal particle, usually organic in nature, composed of aggregates of large molecules, e.g., in soaps and surfactants. For aqueous solutions, the hydrophilic end of the molecule is on the surface of the micelle, while the hydrophobic end (often a hydrocarbon chain) points toward the centre.
saponification → saponifikacija
Saponification is a proces of hydrolysis of esters using hot sodium hydroxide solution to produce the salt of a carboxylic acid. Saponification usually refers to the hydrolysis of esters of fatty acids to manufacture soaps.
water softener → omekšivač vode
Water softeners are substances which help remove constant water hardness. It reacts with calcium and magnesium salts, creating compounds that do not react with soap.
triols → trioli
Trihydric alcohols (i.e. Triols) are organic compounds containing three hydroxyl groups. The simplest trihydric alcohol is 1,2,3-propane-triol, CH2(OH)CH(OH)CH2(OH), which is also known as glycerol (from the Greek glykys meaning sweet) or glycerin. Glycerol is commercially produced by the hydrolysis of fats.
Glycerol is a by-product in the soap industry and is recovered by suitable means.
water hardness → tvrdoća vode
Hardness is defined as the concentrations of calcium and magnesium ions expressed in terms of calcium carbonate. These minerals in water can cause some everyday problems. They react with soap and produce a deposit called soap curd that remains on the skin and clothes and, because it is insoluble and sticky, cannot be removed by rinsing.
Hard water may also shorten the life of plumbing and water heaters. When water containing calcium carbonate is heated, a hard scale is formed that can plug pipes and coat heating elements. Scale is also a poor heat conductor. With increased deposits on the unit, heat is not transmitted to the water fast enough and overheating of the metal causes failure. Build-up of deposits will also reduce the efficiency of the heating unit, increasing the cost of fuel.
There are two types of water hardness, temporary and permanent.
Temporary Hardness is due to the bicarbonate ion, HCO3-, being present in the water. This type of hardness can be removed by boiling the water to expel the CO2, as indicated by the following equation:
Permanent hardness is due to calcium and magnesium nitrates, sulphates, and chlorides etc. This type of hardness cannot be eliminated by boiling.
| Water supply classification | |
|---|---|
| Hardness | Concentration of Calcium carbonate (mg/L) |
| Soft Water | 0 to 75 |
| Medium Hard Water | 75 to 150 |
| Hard Water | 150 to 300 |
| Very Hard Water | over 300 |
Citing this page:
Generalic, Eni. "Sapun." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table