reversible reaction → reverzibilna reakcija
Reversible reaction is a chemical reaction that can proceed in both the forward and backward directions. When reversible reactions reach equilibrium the forward and reverse reactions are still happening but at the same rate, so the concentrations of reactants and products do not change. A reversible reaction is denoted by a double arrow pointing both directions in a chemical equation.
rhenium → renij
Rhenium was discovered by Walter Noddack, Ida Tacke and Otto Berg (Germany) in 1925. The origin of the name comes from the Latin word Rhenus meaning river Rhine. It is rare and costly, dense, silvery-white metal. Tarnishes in moist air. Resists corrosion and oxidation. Dissolves in nitric and sulfuric acids. Has a very high melting point. Rhenium is found in small amounts in gadolinite and molybdenite. Mixed with tungsten or platinum to make filaments for mass spectrographs. Its main value is as a trace alloying agent for hardening metal components that are subjected to continuous frictional forces.
rubidium → rubidij
Rubidium was discovered by Robert Bunsen and Gustav Kirchhoff (Germany) in 1861. The origin of the name comes from the Latin word rubidius meaning dark red or deepest red. It is soft, silvery-white, highly reactive metal. Ignites in air. Reacts violently with water or oxidants. Rubidium occurs abundantly, but so widespread that production is limited. Usually obtained from lithium production. Used as a catalyst, photocells and vacuum and cathode-ray tubes.
rutherfordium → ruthefordij
Rutherfordium was discovered by workers at the Nuclear Institute at Dubna (USSR) and by workers at the University of California, Berkeley (USA) in 1964. Name in honour of Lord Rutherford, the physicist and chemist from New Zealand. It is synthetic radioactive metal. Rutherfordium was made by bombarding californium-249 with beams of carbon-12 and 13. Six isotopes of rutherfordium have so far been identified. Rutherfordium-261, the longest-lived, has a half-life of 62 seconds.
samarium → samarij
Samarium was discovered by Paul Emile Lecoq de Boisbaudran (France) in 1879. Named after the mineral samarskite. It is silvery rare earth metal. Stable in dry air. Oxide coating forms on surfaces exposed to moist air. Metal ignites and burns readily. Reacts with water. Samarium is found with other rare earths in monazite sand. It is used in the electronics and ceramics industries. It is easily magnetized and very difficult to demagnetise. This suggests important future applications in solid-state and superconductor technologies.
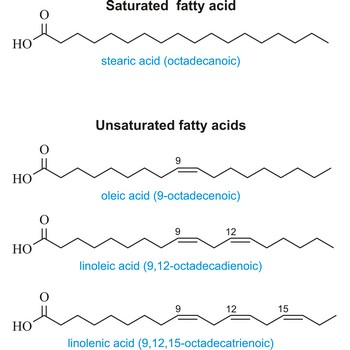
saturated fatty acid → zasićena masna kiselina
Saturated fatty acid is a fatty acid carrying the maximum possible number of hydrogen atoms (It doesn’t have any double bounds in the alkyl chain). The most important of these are:
| Butyric (butanoic acid) | CH3(CH2)2COOH |
| Lauric (dodecanoic acid) | CH3(CH2)10COOH |
| Myristic (tetradecanoic acid) | CH3(CH2)12COOH |
| Palmitic (hexadecanoic acid) | CH3(CH2)14COOH |
| Stearic (octadecanoic acid) | CH3(CH2)16COOH |
| Arachidic (eicosanoic acid) | CH3(CH2)18COOH |
scandium → skandij
Scandium was discovered by Lars Fredrik Nilson (Sweden) in 1879. The origin of the name comes from the Latin word Scandia meaning Scandinavia. It is fairly soft, silvery-white metal. Burns easily. Tarnishes readily in air. Reaction with water releases hydrogen. Reacts with air and halogens. Scandium occurs mainly in the minerals thortveitile (~34 % scandium) and wiikite. Also in some tin and tungsten ores. Pure scandium is obtained as a by-product of uranium refining. Scandium metal is used in some aerospace applications. Scandium oxide (Sc2O2) is used in the manufacture of high-intensity electric lamps. Scandium iodide (ScI3) is used in lamps that produce light having a colour closely matching natural sunlight.
Schrodinger equation → Schrodingerova jednadžba
Schrödinger equation is the basic equation of wave mechanics which, for systems not dependent on time, takes the form:
where Ψ is the wavefunction, V is the potential energy expressed as a function of the spatial coordinates, E its total energy, ![]() 2 is the Laplacian operator, h is Planck’s constant, and m is the mass.
2 is the Laplacian operator, h is Planck’s constant, and m is the mass.
sediment → sediment
1. Sediment is a fragmental material that originates from weathering of rocks and is transported by, suspended in, or deposited by water or air or is accumulated in beds by other natural agencies. When solidified, sediments form sedimentary rocks.
2. Strictly, sediment is a solid material that has settled down from a state of suspension in a liquid.
semiconductor → poluvodič
Semiconductor is a material in which the highest occupied energy band (valence band) is completely filled with electrons at T = 0 K, and the energy gap to the next highest band (conduction band) ranges from 0 to 4 or 5 eV. With increasing temperature electrons are excited into the conduction band, leading to an increase in the electrical conductivity.
Citing this page:
Generalic, Eni. "Sửa báo cáo khoản vay nước ngoài." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table