lawrencium → lawrencij
Lawrencium was discovered by Albert Ghiorso, Torbjorn Sikkeland, Almon E. Larsh and Robert M. Latimer (USA) in 1961. Named in honour of Ernest O. Lawrence, inventor of the cyclotron. It is synthetic radioactive metal. Lawrencium was produced by bombarding a mixture of three isotopes of californium with boron-10 and boron-11 ions. Eight isotopes of lawrencium have been synthesized to date, with the longest-lived being lawrencium-256, which has a half-life of about 30 seconds.
regeneration → regeneracija
Regeneration is the process of restoring an ion exchange medium to a usable state after exhaustion. The cation exchanger is normally regenerated with hydrochloric acid and the anion exchanger with sodium hydroxide.
reverse reaction → povratna reakcija
Reverse reaction is a reaction in backward directions, reaction (in the reversible reaction) in which original reactants emerge again from a products. It goes from right to left.
ligand → ligand
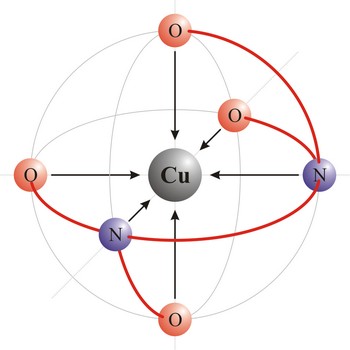
Ligand is an ion (F-, Cl-, Br-, I-, S2-, CN-, NCS-, OH-, NH2-) or molecule (NH3, H2O, NO, CO) that donates a pair of electrons to a metal atom or ion in forming a coordination complex. The main way of classifying ligands is by the number of points at which they are attached to, or bound to, the metal center. This is the denticity. Ligands with one potential donor atom are monodentate. Polydentate ligand is a ligand that is attached to a central metal ion by bonds from two or more donor atoms. Ligands with more than one potential donor atom are known as ambidentate, such as the thiocyanate ion, NCS-, which can bind to the metal center with either the nitrogen or sulphur atoms. Chelating ligands are those polydentate ligands which can form a ring including the metal atom.
lime → živo vapno
Lime (or quicklime) is the common name for calcium oxide (CaO). It is manufactured from limestone, CaCO3, by heating it to a high temperature (about 1 000 °C). At this temperature carbon dioxide, CO2, is released from the limestone creating calcium oxide, CaO.
A further process involves adding water in a process known as hydrating, which produces hydrated, or slaked lime [Ca(OH)2].
limestone → vapnenac
Limestone is a sedimentary rock composed primarily of calcium carbonate in the form of the mineral calcite.. Some 10 % to 15 % of all sedimentary rocks are limestones. Limestone is usually organic, but it may also be inorganic. Calcium carbonate may have been directly precipitated from the sea-water or by the lithification of coral reefs, marine organism shells, or marine organism skeletons.
Citing this page:
Generalic, Eni. "Sửa báo cáo khoản vay nước ngoài." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table