carbon → ugljik
Carbon has been known since ancient times. The origin of the name comes from the Latin word carbo meaning charcoal. Graphite form of carbon is a black, odourless, slippery solid. Graphite sublimes at 3825 °C. Diamond form is a clear or colored; an extremely hard solid. C60 is Buckminsterfullerine. Carbon black burns readily with oxidants. Carbon is made by burning organic compounds with insufficient oxygen. There are close to ten million known carbon compounds, many thousands of which are vital to organic and life processes. Radiocarbon dating uses the carbon-14 isotope to date old objects.
carboxylic acids → karboksilne kiseline
Carboxylic acids are organic compounds characterized by the presence of one or more RC(=O)OH groups (the carboxyl group). In the systematic chemical nomenclature carboxylic acids names end in the suffix -oic (e.g. ethanoic acids, CH3COOH). The carbon of the terminal group being counted as part of the chain. They are generally weak acids. Carboxylic acids include a large and important class of fatty acids and may be either saturated or unsaturated. There are also some natural aromatic carboxylic acids (benzoic, salicylic).
catalyst → katalizator
Catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. Catalysts that have the same phase as the reactants are homogenous catalysts (e.g. enzymes in biochemical reactions). Those that have a different phase are heterogeneous catalyst (e.g. metals or oxides used in gas reactions).
The catalyst provides an alternative pathway by which the reaction can proceed, in which the activation energy is lower. In thus increases the rate at which the reaction comes to an equilibrium, although it does not alter the position of the equilibrium.
cathode → katoda
Cathode is a negative electrode of an electrolytic cell to which positively charged ions (cations) migrate when a current is passed as in electroplating baths.
In a primary or secondary cell (battery or accumulator) the cathode is the electrode that spontaneously becomes negative during discharge, and form which therefore electrons emerge.
In vacuum electronic devices electrons are emitted by the cathode and flow to the anode.
Lewis base → Lewisova baza
Lewis base is an agent capable of donating a pair of electrons to form a coordinate bond.
neutral substance → neutralna tvar
Neutral substance is a substance that shows no acid or base properties, has an equal number of hydrogen and hydroxyl ions and does not change the colour of litmus-paper.
sublimation → sublimacija
Sublimation is the conversion of a substance from its solid form directly to its gaseous form without the intervening liquid form. Dry ice (frozen carbon dioxide) sublimates at normal room temperature.
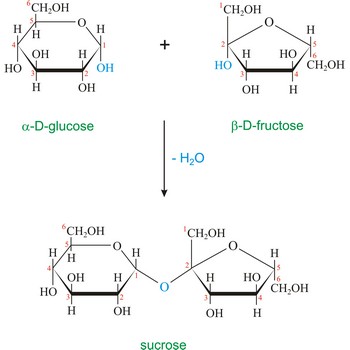
sucrose → saharoza
Sucrose (saccharose), or ordinary table sugar, is a disaccharide in which α-D-glucopyranose and β-D-fructofuranose are joined at their anomeric carbons by a glycosidic bond. There are no hemiacetals remaining in the sucrose and therefore sucrose is not a reducing sugar and does not exhibit mutarotation. Sugar is a white crystalline sweet compound found in many plants and extracted from sugar cane and sugar beet. It is used as a sweetening agent in food and drinks. If heated to 200 °C, sucrose becomes caramel. When sucrose is hydrolyzed it forms an equimolar mixture of glucose and fructose. This mixture of monosaccharides is called invert sugar. Honeybees have enzymes called invertases that catalyze the hydrolysis of sucrose. Honey, in fact, is primarily a mixture of glucose, fructose, and sucrose.
sugar → šećer
Sugar is any of a group of water-soluble carbohydrates of relatively low molecular weight and typically having a sweet taste. The group comprises mainly monosaccharides (glucose, fructose, galactose), disaccharides (sucrose, lactose, maltose), and trisaccharides (raffinose). Many monosaccharides and disaccharides fairly commonly found in nature bear names reflecting the source from which they were first isolated. For example, glucose is also known as grape sugar, lactose as milk sugar, and maltose as malt sugar. In everyday usage, the name is often used to refer specifically to sucrose (table sugar, cane sugar, beet sugar).
sulfur → sumpor
Sulfur has been known since ancient times. The origin of the name comes from the Sanskrit word sulvere meaning sulphur; also from the Latin word sulphurium meaning sulphur. It is pale yellow, odourless, brittle solid, which is insoluble in water but soluble in carbon disulfide. Sulfur is found in pure form and in ores like cinnabar, galena, sphalerite and stibnite. Pure form is obtained from underground deposits by the Frasch process. Used in matches, gunpowder, medicines, rubber and pesticides, dyes and insecticides. Also for making sulfuric acid (H2SO4).
Citing this page:
Generalic, Eni. "Sửa báo cáo khoản vay nước ngoài." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table