graphite → grafit
Graphite is an allotrope of carbon. The atoms are arranged in layers as a series of flat, hexagonal rings. Graphite is a good conductor of heat and electricity. The layers cleave easily, making graphite useful as a solid lubricant. A process to make pure synthetic graphite was invented by the American chemist Edward Goodrich Acheson (1856–1931). The process consists of heating a mixture of clay (aluminum silicate) and powdered coke (carbon) in an iron bowl. The reaction involves the production of silicon carbide, which loses silicon at 4150 °C to leave graphite.
half-life of a reactant → poluživot reaktanta
For a given reaction the half-life, t1/2, of a reactant is the time required for its concentration to reach a value that is the arithmetic mean of its initial and final (equilibrium) value.
Half-life is constant for first-order reactions.
Half-life is not constant for second-order reactions but rather it varies with initial concentration and k.
Hesse’s law → Hessov zakon
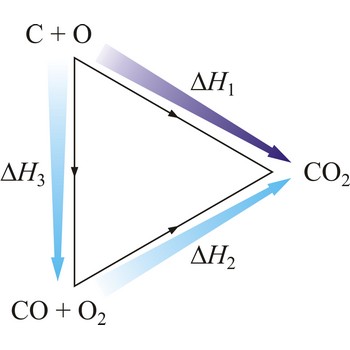
Hesse’s law says that reaction heat of some chemical change does not depend on the way in which the reaction is conducted, but only on starting and ending system state. Hesse’s law is also known as the law of constant heat summation. Hesse’s law is also known as the law of constant heat summation. The law was first put forward in 1840 by the Swiss-born Russian chemist Germain Henri Hess (1802-1850).
Hesse’s law can be used to obtain thermodynamic data that cannot be measured directly. For example, it is very difficult to control the oxidation of graphite to give pure CO. However, enthalpy for the oxidation of graphite to CO2 can easily be measured. So can the enthalpy of oxidation of CO to CO2. The application of Hess’s law enables us to estimate the enthalpy of formation of CO.
| C(s) + O2(g) →← CO2(g) | ΔrH1 = -393 kJ mol-1 |
| CO(g) + 1/2O2(g) →← CO2(g) | ΔrH2 = -283 kJ mol-1 |
| C(s) + 1/2O2(g) →← CO(g) | ΔrH3 = -110 kJ mol-1 |
The equation shows the standard enthalpy of formation of CO to be -110 kJ/mol.
hydrogen → vodik
Hydrogen was discovered by Sir Henry Cavendish (England) in 1766. The origin of the name comes from the Greek words hydro and genes meaning water and generate. It is colourless, odourless gas, burns and forms explosive mixtures in air. Reacts violently with oxidants. Hydrogen is the most abundant element in the universe. Commercial quantities of hydrogen are produced by reacting superheated steam with methane or carbon. In lab work from reaction of metals with acid solutions or electrolysis. Most hydrogen is used in the production of ammonia and in metal refining. Also used as fuel in rockets. Its two heavier isotopes (deuterium and tritium) used respectively for nuclear fusion.
Schiff base → Schiffova baza
Schiff base is a class of compounds derived by the chemical reaction (condensation) of aldehydes or ketones with aromatic amines, for example
They were named after the German chemist Hugo Schiff (1834-1915).
spontaneously combustible → samozapaljivi materijal
Spontaneously combustible materials are materials that can ignite without an external source of heat. Heat sufficient to reach the ignition temperature may be generated by reaction with oxygen in the air, by the absorption of moisture, from heat generated during processing, or even from radioactive decay.
hydrolysis → hidroliza
Hydrolysis is a chemical reaction in which water reacts with another substance to form two or more new substances. This involves ionisation of the water molecule, as well as splitting of the compound hydrolysed, e.g.
Examples are conversion of starch to glucose by water in the presence of suitable catalysts and a reaction of the ions of a dissolved salt to form various products, such as acids, complex ions, etc.
Ilkovic equation → Ilkovičeva jednadžba
Ilkovic equation is a relation used in polarography relating the diffusion current (id) and the concentration of the depolarizer (c), which is the substance reduced or oxidized at the dropping mercury electrode. The Ilkovic equation has the form
Where k is a constant which includes Faraday constant, π and the density of mercury, and has been evaluated at 708 for max current and 607 for average current, D is the diffusion coefficient of the depolarizer in the medium (cm2/s), n is the number of electrons exchanged in the electrode reaction, m is the mass flow rate of Hg through the capillary (mg/sec), and t is the drop lifetime in seconds, and c is depolarizer concentration in mol/cm3.
The equation is named after the scientist who derived it, the Slovak chemist, Dionýz Ilkovič 1907-1980).
Citing this page:
Generalic, Eni. "Reverse reaction." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table