Einstein, Albert → Einstein, Albert
Albert Einstein (1879-1955) is a German born American physicist, who took Swiss nationality in 1901. A year later he went to work in the Bern patent office. In 1905. he published five enormously influential papers, one on Brownian movement, one on the photoelectric effect, one on the special theory of relativity, and one on energy and inertia (which included the famous expression E = mc2). In 1915 he published the general theory of relativity, concerned mainly with gravitation. In 1921 he was awarded the Nobel Prize. In 1933, as a Jew, Einstein decided to remain in the USA (where he was lecturing), as Hitler had come to power. For the remainder of his life he sought a unified field theory. In 1939 he informed president Roosevelt that an atom bomb was feasible and that. Germany might be able to make one.
electronegativity → elektronegativnost
Electronegativity is a parameter originally introduced by L. Pauling which describes, on a relative basis, the power of an atom to attract electrons. For example, in hydrogen chloride, the chlorine atom is more electronegative than the hydrogen and the molecule is polar, with a negative charge on the chlorine atom.
There are various ways of assigning values for the electronegativity of an element. Pauling electronegativities are based on bond dissociation energies using a scale in which fluorine, the most electronegative element, has the value 4 and francium, the lowest electronegative element, has the value 0.7.
fuel cell → gorivi članak
Fuel cell is a device that converts chemical energy into electrical energy. It is different from a battery in that the energy conversion continues as long as fuel and oxidising agent are fed to the fuel cell; that is, in principle indefinitely. (A battery is manufactured with a limited amount of chemicals, and it is exhausted when all the chemicals have reacted.) It is a galvanic cell where spontaneous chemical reactions occur at the electrodes. The fuel is oxidised at the anode, and the oxidising agent (almost always oxygen or air) is reduced at the cathode. Presently, the most commonly used fuel is hydrogen. More conventional fuels (e.g., petrol or natural gas) must be converted (reformed) into hydrogen before they can be utilised in a fuel cell.
Some fuel cells employ an aqueous solution as electrolyte, that can be either acidic or basic (alkaline), or an ion-exchange membrane soaked in aqueous solution can act as the electrolyte. These fuel cells operate at relatively low temperatures (from room temperature to not much above the boiling point of water). Some fuel cells employ molten salts (especially carbonates) as electrolytes and have to operate at temperatures of several hundred degrees centigrade (Celsius). Others employ ionically conductive solids as electrolyte and must operate close to 1 000 °C.
protein → bjelančevina
Proteins are natural organic compounds of animal or herbal origin, essential in diet. They are natural polymers developed from a crowd of interconnecting monomers of amino acids, with relative molecular masses amounting up to a few million.
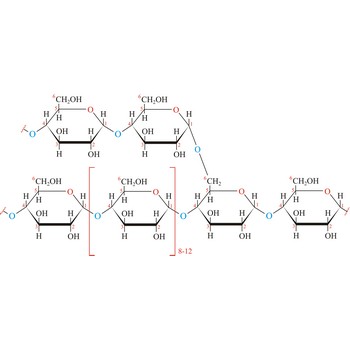
glycogen → glikogen
Glycogen (animal starch) is a polysaccharide that serves the same energy storage function in animals that starch serves in plants. Dietary carbohydrates not needed for immediate energy are converted by the body to glycogen for long term storage (principally in muscle and liver cells). Like amylopectin found in starch, glycogen is a polymer of α(1→4)-linked subunits of glucose, with α(1→6)-linked branches. Glycogen molecules are larger than those of amylopectin (up to 100 000 glucose units) and contain even more branches. Branch points occur about every 10 residues in glycogen and about every 25 residues in amylopectin. The branching also creates lots of ends for enzyme attack and provides for rapid release of glucose when it is needed.
Knudsen burette → Knudsenova bireta
Knudsen's automatic bulb-burette, developed by the Danish physicist Martin Knudsen (1871-1949), is designed in a way that even routine field analysis in a boat laboratory would provide highly accurate measurements. The burette is filled with a mixture of silver nitrate from reservoir R, located above the burette, by opening the A valve. When the solution crosses the three-way C valve the A valve is closed preventing further solution flow in to the burette. Any extra solution is caught in the W bowl. Turn the C valve, which marks the zero on the scale, in order to allow atmospheric air to enter the burette. Since most open-ocean samples lie in a relatively small chlorinity range, the burette is designed so that much of its capacity is in the bulb (B). This allows the titration to be quick (by quickly releasing contents from the B area) and reduces the error that occurs from the slow drainage along the inner wall of the burette.
Each millimeter is divided in to twenty parts (double millimeter division of the Knudsen burette) which allows for highly accurate measurements (the scale is read up to a precision of 0.005 mL). From 0 to 16 the burette isn't divided, that usually starts from 16 and goes until 20.5 or 21.5. A single double millimeter on a Knudsen burette scale corresponds to one permille of chloride in the seawater sample. This burette can be used for titration of water from all of the oceans and seas, with the exemptions being areas with very low salinity (e.g. the Baltic Sea) and river estuaries which require the use of normal burettes.
lead-acid battery → olovni akumulator
Lead-acid battery is a electrical storage device that uses a reversible chemical reaction to store energy. It was invented in 1859 by French physicist Gaston Planté. Lead-acid batteries are composed of a lead(IV) oxide cathode, a sponge metallic lead anode and a sulphuric acid solution electrolyte.
In charging, the electrical energy supplied to the battery is changed to chemical energy and stored. The chemical reaction during recharge is normally written:
In discharging, the chemical energy stored in the battery is changed to electrical energy. During discharge, lead sulfate (PbSO4) is formed on both the positive and negative plates. The chemical reaction during discharge is normally written:
Lead acid batteries are low cost, robust, tolerant to abuse, tried and tested. For higher power applications with intermittent loads however, they are generally too big and heavy and they suffer from a shorter cycle life.
lutetium → lutecij
Lutetium was discovered by Georges Urbain (France) and independently by Carl Auer von Welsbach (Austria) in 1907. The origin of the name comes from the Greek word Lutetia meaning Paris. It is silvery-white and relatively stable in air, rare earth metal. Lutetium is found with ytterbium in gadolinite and xenotime. Stable lutetium nuclides can be used as catalysts in cracking, alkylation, hydrogenation, and polymerization.
macromolecule → makromolekule
Macromolecule is a molecule of high relative molecular mass (molecular weight), the structure of which essentially comprises the multiple repetitions of units derived, actually or conceptually, from molecules of low relative molecular mass. The types of macromolecules are natural and synthetic polymers, carbohydrates, lipids, proteins etc. Cellulose is a polysaccharide that is made up of hundreds, even thousands of glucose molecules strung together.
magnetic permeability → magnetska permeabilnost
Magnetic permeability (μ), also called permeability, is a constant of proportionality that exists between magnetic induction and magnetic field intensity. This constant is equal to approximately μo = 1.257×10-6 H/m in a vacuum.
Magnetic permeability is often expressed in relative, rather than in absolute, terms. If μ represents the permeability of the substance in question, then the relative permeability, μr, is given by:
Citing this page:
Generalic, Eni. "Relativna vlažnost." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table