limiting reactant → mjerodavni reaktant
Limiting reactant is a reactant in a chemical reaction that limits the amount of product that can be formed. The reaction will stop when the entire limiting reagent is consumed. These other reactants are present in excess.
half-life of a reactant → poluživot reaktanta
For a given reaction the half-life, t1/2, of a reactant is the time required for its concentration to reach a value that is the arithmetic mean of its initial and final (equilibrium) value.
Half-life is constant for first-order reactions.
Half-life is not constant for second-order reactions but rather it varies with initial concentration and k.
activated complex → aktivirani kompleks
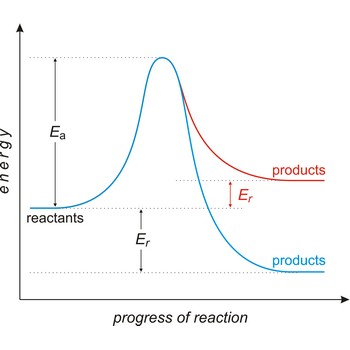
Activated complex is an intermediate structure formed in the conversion of reactants to products. The activated complex is the structure at the maximum energy point along the reaction path; the activation energy is the difference between the energies of the activated complex and the reactants.
activation energy → energija aktivacije
Activation energy (Ea) is the energy that must be added to a system in order for a process to occur, even though the process may already be thermodynamically possible. In chemical kinetics, the activation energy is the height of the potential barrier separating the products and reactants. It determines the temperature dependence on the reaction rate.
active site → aktivno mjesto
Active site is a pocket or crevice on an enzyme molecule that fits reactant molecules like a hand in a glove. The active site lowers the activation energy for reaction
addition reactions → reakcije adicije
Addition reactions are normally occur with unsaturated compounds and involve the addition of one molecule (called the reactant) across the unsaturated bond (i.e. the double bond or the triple bond) of another molecule (called the substrate) to give a single product, formed by the combination of both reacting molecules.
For example, bromine adds across the double bond of ethene in an addition reaction to form dibromoethane.
average rate of reaction → prosječna brzina reakcije
Average rate of reaction is calculated in a way that a total change of reactants and products concentration is divided with time which is needed for reaction to end.
chemical balance → kemijska ravnoteža
Chemical balance is a degree of reversible reaction in a closed system, when the forward and backward reaction happen at same rates and their effects annul each other, while the concentration of reactants and products stays the same.
chemical equation equalization → izjednačavanje kemijske jednadžbe
Chemical equation equalization is determining values of stechiometric coefficients of reactants and products in a chemical equation in a way that the number of atoms of each element is equal before and after the reaction.
Citing this page:
Generalic, Eni. "Reaktant." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table