seawater → more
Seawater is a complex mixture of 96.5 % water, 3.5 % salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates, and a few atmospheric gases. The world's oceans cover nearly 71 % (361 840 000 km2) of the Earth's surface (510 100 000 km2), with an average depth of 3 682.2 m.
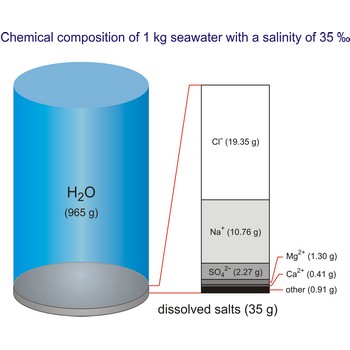
The density of seawater is higher than that of fresh water because of its higher salinity. Seawater's freezing point is lower than that of pure water and its boiling point is higher. The average salinity of the ocean is 35 ‰, which means that for every kilograms of water, there are 35 g of salt. The relative abundance of the major salts in seawater are constant regardless of the ocean. Only six elements and compounds comprise about 99 % of sea salts: chlorine (Cl-), sodium (Na+), sulfur (SO42-), magnesium (Mg2+), calcium (Ca2+), and potassium (K+).
salinity → salinitet
Salinity (S) is a measure of the quantity of dissolved salts in seawater. It is formally defined as the total amount of dissolved solids in seawater in parts per thousand (‰) by weight when all the carbonate has been converted to oxide, the bromide and iodide to chloride, and all organic matter is completely oxidized.
Chlorinity is the oldest of the salinity measures considered and is still a corner-stone in the study of dissolved material in seawater. Based on the principle of constant relative proportions it provides a measure of the total amount of dissolved material in seawater in terms of the concentration of halides. The relationship between chlorinity (Cl) and salinity as set forth in Knudsen’s tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
Practical Salinity (SP) was introduced as a replacement for Chlorinity. Practical Salinity is is relatively easy to measure using standard conductometers, measurements are more precise and less time consuming than measurements of Chlorinity and accurate measurements can even be made in situ. Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution).
Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". For most purposes one can assume that the psu and the ‰, are synonymous.
The global average salinity of ocean waters is about 35 ‰, that is, about 35 g of solid substances are dissolved in 1 kg of seawater.
sedimentary rocks → sedimentne stijene
Sedimentary Rocks are formed by the accumulation and subsequent consolidation of sediments into various types of rock. There are three major types of sedimentary rocks:
Biogenic sedimentary rocks are formed from organic processes when organisms use materials dissolved in water to build a shell or other skeletal structure.
Clastic sedimentary rocks are composed directly of the sediments or fragments from other rocks.
Chemical sedimentary rocks are formed through evaporation of a chemical rich solution.
Based on their sizes, sediment particles are classified, based on their size, into six general categories:
- boulder (>256 mm)
- cobble (64 - 256 mm)
- gravel (2 - 64 mm)
- sand (1/16 - 2 mm)
- silt (1/256 - 1/16 mm)
- clay (<1/256 mm)
sedimentation → sedimentiranje
Sedimentation is a process of separating specifically heavier, suspended matter, than the solution is. Solid matter settles on the bottom of the vessel and the liquid above it is poured off. The settling zone is the largest portion of the sedimentation basin. This zone provides the calm area necessary for the suspended particles to settle. The sludge zone, located at the bottom of the tank, provides a storage area for the sludge before it is removed for additional treatment or disposal.
smoke → dim
Smoke is a fine suspension of solid particles in a gas. In general smoke particles range downward from about 5 μm in diameter to less than 01 μm in diameter. Smoke generally refers to a visible mixture of products given off by the incomplete combustion of an organic substance such as wood, coal, fuel oil etc. This airborne mixture general contains small particles (dusts) of carbon, hydrocarbons, ash etc. as well as vapors such as carbon monoxide, carbon dioxide, and water vapor.
Snell’s law → Snellov zakon
When a light ray comes on a boundary between two transparent media, it will be partly reflected and partly refracted. Both rays, reflected and refracted ray, lay in the plane of incidence. The angle of reflection is equal to the angle of incidence. The angle of refraction (Θ2) is related to the angle of incidence (Θ1) via Snell’s law:
where n1 and n2 are dimensionless constants - indexes of refraction of the two media.
sol → sol
Sols are dispersions of small solid particles in a liquid. The particles may be macromolecules or may be clusters of small molecules. Lyophobic sols are those in which there is no affinity between the dispersed phase and the liquid (e.g. silver chloride dispersed in water). Lyophobic sols are inherently unstable, in time the particles aggregate, and form a precipitate. Lyiophilic sols, on the other hand, are more like true solutions in which the solute molecules are large and have an affinity for the solvent (e.g. starch in water). Association colloids are systems in which the dispersed phase consists of clusters of molecules that have lyophobic and lyophilic parts (e.g. soap in water).
solid state → čvrsto agregatno stanje
Solid state is characterised by a constant shape and volume. Particles are placed very close to one another and have efect one on another with great attraction forces. Solid bodies do not assume the shape of the container in which they are put.
solution composition → sastav otopine
Solutions are homogenous mixtures of several components. The component which is found in a greater quantity is called the solvent and the other components are called solutes. Quantitative composition of a solution can be expressed by concentration (amount, mass, volume and number), by fraction (amount, mass, and volume), ratio (amount, mass, and volume) and by molality. Amount, mass, and volume ratio are numerical, nondimensional units and are frequently expressed as percentage (% = 1/100), promile (‰ = 1/1000) or parts per million (ppm = 1/1 000 000). If it is not defined, it is always related to the mass ratio.
spin → spin
Spin is the intrinsic angular momentum of an elementary particle, or system of particles such as nucleus, that is also responsible for the magnetic moment; or, a particle or nucleus possessing such a spin. The spins of nuclei have characteristic fixed values. Pairs of neutrons and protons align to cancel out their spins, so that nuclei with an odd number of neutrons and/or protons will have a net non-zero rotational component characterized by a non-zero quantum nuclear spin number.
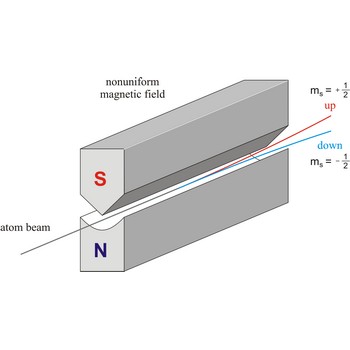
Stern-Gerlach experiment: a beam of silver atoms is split into two beams when it traverses a nonuniform magnetic field. Atoms with spin quantum number ms=+1/2 follow one trajectory, and those with ms=+1/2 follow another.
Citing this page:
Generalic, Eni. "Rúbrica para evaluar un portafolio digital." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table