nucleic acid → nukleinska kiselina
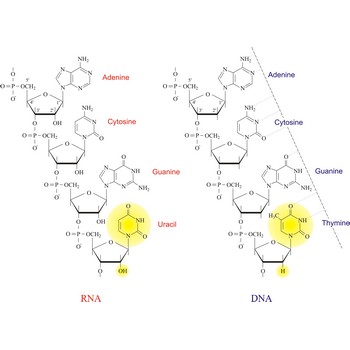
Nucleic acids are a complex, high-molecular-weight biochemical macromolecules composed of nucleotide chains that convey genetic information. The most common nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Each nucleic acid chain is composed of subunits called nucleotides, each containing a sugar, a phosphate group, and nitrogenous base. DNA was first discovered in 1869 by the Swiss biochemist Friedrich Miescher (1844-1895).
Both DNA and RNA contain the two major purine bases adenine (A) and guanine (G) and one of the major pyrimidines, cytosine (C). Of the other two pyrimidines, thymine (T) is found in DNA and uracil (U) is found in RNA. There are two major pentoses in nucleic acids:2'-deoxy-D-ribose in DNA and D-ribose in RNA.
Nucleotides are linked together in both DNA and RNA in a polymeric fashion via covalent bonds. These bonds exist through phosphate-group bridges in which the 5' hydroxyl group of one nucleotide unit is joined to the 3' hydroxyl group of the next nucleotide. RNA is usually a single-stranded molecule, whereas DNA is usually double-stranded.
omega-3 fatty acids → omega-3 masne kiseline
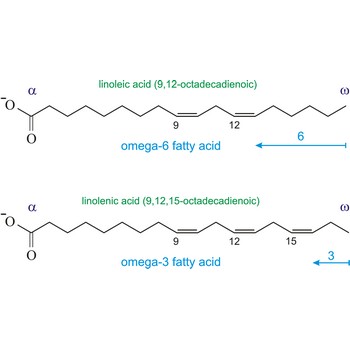
Omega-3 fatty acids are polyunsaturated fatty acids, meaning they contain more than one double bond. The name omega-3 indicates that the first double bond occurs on the third carbon atom (n-3) from the methyl (-CH3) end of the molecule (omega position). The three main omega-3 fatty acids are alpha-linolenic acid (ALA, 18:3n-3), eicosapentaenoic acid (EPA, 20:5n-3), and docosahexaenoic acid (DHA, 22:6n-3). ALA comes from plants. EPA and DHA come from fish.
Similarly, the first double bond in omega-6 fatty acids is located between the sixth and seventh carbon atom (n-6) from the methyl end of the fatty acid (omega end).
Ostwald’s process → Ostwaldov proces
Ostwald’s process is a process by which the nitric acid can be obtained in three degrees. In the first stage ammonia and oxygen react (with platinum-rhodium as a catalyst), whereby the nitrogen monoxide and water emerge
In the second stage nitrogen monoxide reacts with oxygen whereby nitrogen dioxide emerges
and in the third stage nitrogen dioxide dissolves in water, in the presence of air, giving the nitric acid
Ostwald’s viscometer → Ostwaldov viskozimetar
Ostwald viscometer, also known as U-tube viscometer or capillary viscometer is a device used to measure the viscosity of the liquid with a known density. The method of determining viscosity with this instrument consists of measuring the time for a known volume of the liquid (the volume contained between the marks A and B) to flow through the capillary under the influence of gravity. Ostwald viscometers named after the German chemist Wilhelm Ostwald (1853-1932).
The instrument must first be calibrated with materials of known viscosity such as pure (deionized) water. Knowing the value of viscosity of one liquid, one can calculate the viscosity of other liquid.
where η1 and η2 are viscosity coefficients of the liquid and water, and ρ1 and ρ2 are the densities of liquid and water, respectively.
picnometer → piknometar
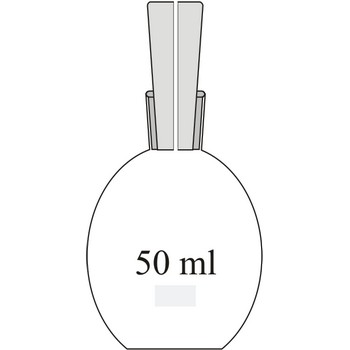
Picnometer is a special glass flask which is used for determining a relative density of liquids using the weight of a known volume. It usually has a glass faceted cork which is pierced in the centre like a thin capillary through which surplus of liquid runs out.
protactinium → protaktinij
Protactinium was discovered by Otto Hahn (Germany) and Lise Meitner (Austria) in 1917. The origin of the name comes from the Greek word protos meaning first. It is very rare, silvery-white, extremely radioactive metal. Resists alkalis; reacts with oxygen and acids. Attacked by steam. Highly radiotoxic. Protactinium is extremely toxic and must be handled with great care. Protactinium does not occur in nature. Found among fission products of uranium, thorium and plutonium.
radon → radon
Radon was discovered by Friedrich Ernst Dorn (Germany) in 1900. The origin of the name is variation of the name of element radium; radon was called niton at first, from the Latin word nitens meaning shining. It is colourless, odourless radioactive, heavy, noble gas. Chemically inert and non-flammable. Highly radiotoxic. Carcinogen by inhalation. Radon is formed from the decay of radium in the earths crust. Used to treat some forms of cancer.
rare earth elements → elementi rijetkih zemalja
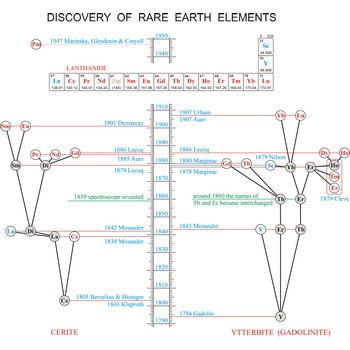
Rare earth elements (metals) are the elements scandium (Sc), yttrium (Y), and the lanthanides (La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu). These elements got their name from the fact that chemists first isolated them in their oxide forms. These oxides somewhat resemble calcium, magnesium and aluminium oxides, sometimes called common earths. Do you want to know more?
significant figures → značajne znamenke
Measurements are not infinitely accurate: we must estimate measurement uncertainty. The number of significant figures is all of the certain digits plus the first uncertain digit.
Rules for significant figures:
- Disregard all initial zeros.
- Disregard all final zeros unless they follow a decimal point.
- All remaining digits including zeros between nonzero digits are significant.
| 0.0023 | has two significant figures |
| 0.109 | has three significant figures |
| 2.00 | has three significant figures |
| 70 | has one significant figure |
In addition and subtraction, the number of significant figures in the answer depends on the original number in the calculation that has the fewest digits to the right of the decimal point.
In multiplication and division, the number of significant figures in a calculated result is determined by the original measurement that has the fewest number of significant digits.
In a logarithm of a number, keep as many digits to the right of the decimal point as there are significant figures in the original number.
In an antilogarithm of a number, keep as many digits as there are digits to the right of the decimal point in the original number.
Soxhlet extractor → Soxhletov ekstraktor
Soxhlet extractor is a laboratory apparatus designed to extract substances with a low solubility in the extracting solvent. The method described by the German chemist Franz von Soxhlet (1848-1926) in 1879 is the most commonly used example of a semi-continuous method applied to extraction of lipids from foods. In the Soxhlet extractor, the sample soaks in hot solvent that is periodically siphoned off, distilled and returned to the sample. During each cycle, a portion of the non-volatile compound dissolves in the solvent. After many cycles the desired compound is concentrated in the distillation flask. The solvent in the flask is then evaporated and the mass of the remaining lipid is measured.
Citing this page:
Generalic, Eni. "Prvi zakon termodinamike." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table