diatomaceous earth → dijatomejska zemlja
Diatomaceous earth is a naturally occurring siliceous sedimentary mineral compound from microscopic skeletal remains (frustules) of diatoms, unicellular aquatic plants of microscopic size. Their fossilized remains are called diatomite and contains approximately 3000 diatom frustules per cubic millimetre.
Diatomite is relatively inert and has a high absorptive capacity, large surface area, and low bulk density. It consists of approximately 90 % silica, and the remainder consists of compounds such as aluminum and iron oxides. The fine pores in the diatom frustules make diatomite an excellent filtering material for waters, beverages, oils, chemicals, as well as many other products.
ecological footprint → ekološki otisak
The Ecological Footprint is defined as the area of productive land and water ecosystems required to produce the resources that the population consumes (food, fiber, timber, energy, and space for infrastructure) and assimilate the wastes that the population produces (CO2 is the only waste product currently included), wherever on Earth the land and water is located. It compares actual throughput of renewable resources relative to what is annually renewed. Non-renewable resources are not assessed, as by definition their use is not sustainable.
Ecological footprints and biocapacity are expressed in global hectares (gha). Each unit corresponds to one hectare of biologically productive space with world average productivity. In U.S. Footprint results are often presented in global acres (ga). One U.S. acre is equal to 0.405 hectares.
Humanity is currently consuming renewable resources at a faster rate than ecosystems can regenerate them and continuing to release more CO2 than ecosystems can absorb. In 2007, humanity's Footprint was 18 billion gha, or 2.7 gha per person. However, the Earth's biocapacity was only 11.9 billion gha, or 1.8 gha per person. This represents an ecological overshoot of 50 per cent. Put another way, people used the equivalent of 1.5 planets to support their activities (more developed countries generally make higher demands on the Earth's ecosystems than poorer, less developed countries).
experiment → eksperiment
Experiment is direct observation under controlled conditions. Most experiments involve carefully changing one variable and observing the effect on another variable (for example, changing temperature of a water sample and recording the change volume that results).
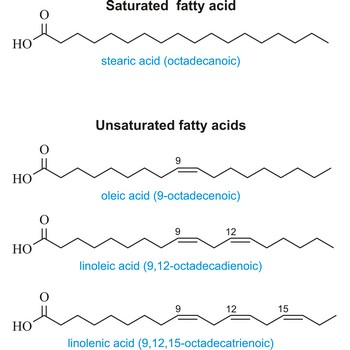
fatty acid → masna kiselina
Fatty acids are aliphatic monocarboxylic acids characterized by a terminal carboxyl group (R-COOH). The higher members of this series of acids occur in nature in the combined form of esters of glycerol (fats), and hence all acids of this family are called fatty acids. Natural fatty acids commonly have a chain of 4 to 28 carbons (usually unbranched and even-numbered), which may be saturated or unsaturated. The most important of saturated fatty acids are butyric (C4), lauric (C12), palmitic (C16), and stearic (C18). The most common unsaturated acids are oleic, linoleic, and linolenic (all C18).
The physical properties of fatty acids are determined by the chain length, degree of unsaturation, and chain branching. Short-chain acids are pungent liquids, soluble in water. As the chain length increases, melting points are raised and water-solubility decreases. Unsaturation and chain branching tend to lower melting points.
freon → freon
Freon (chlorofluorocarbon, CFC) a type of compound in which some or all of the hydrogen atoms of hydrocarbon (usually an alkane) have been replaced by chlorine and fluorine atoms. Most CFC are chemically uncreative and are stable at high temperatures. They are used as aerosol propellants, refrigerants, and solvents, and in the manufacture of rigid packaging foam. CFC because of their chemical inertness, can diffuse unchanged into the upper atmosphere. Here, photochemical reactions cause them to break down and react with ozone. For his reason, their use has been discouraged.
iridium → iridij
Iridium was discovered by Smithson Tennant (England) in 1803. The origin of the name comes from the Latin word iris, meaning rainbow, because its salts are highly colored. It is heavy, brittle, white metal. Unreactive in air, water and acids. Attacked by fused NaOH. Metal ignites and burns readily. Iridium is found in gravel deposits with platinum. Used with osmium to tip gold pen points, to make crucible and special containers. Also to make alloys used for standard weights and measures and heat-resistant alloys. Also as hardening agent for platinum.
Citing this page:
Generalic, Eni. "Prirodni kaučuk." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table