isotope proportion → izotopni omjer
Isotope proportion is a number of atoms of each isotope in a sample of one element. It uses relative masses of isotopes to calculate a relative mass of one element.
joule → džul
Joule (J) is the SI derived unit of energy, work, and heat. The joule is the work done when the point of application of a force of one newton is displaced a distance of one metre in the direction of the force (J = N m). The unit was named after the British scientist James Prescott Joule (1818-1889).
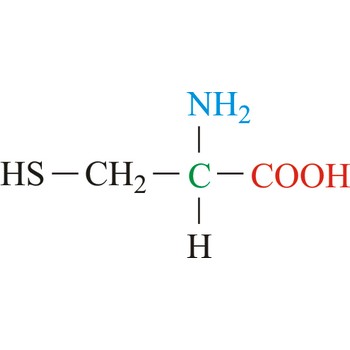
cysteine → cistein
Cysteine is neutral amino acids with polar side chains. Because of its high reactivity, the thiol group of cysteine has numerous biological functions. It serves as a potent nucleophile and metal ligand (particularly for iron and zinc), but is best known for its ability to form disulfide bonds, which often make an important contribution to the stability of extracellular proteins. Cysteine is a non-essential amino acid, which means that it is biosynthesized in humans.
- Abbreviations: Cys, C
- IUPAC name: 2-amino-3-sulfanylpropanoic acid
- Molecular formula: C3H7NO2S
- Molecular weight: 121.16 g/mol
Dalton’s atomic theory → Daltonova atomska teorija
Dalton’s atomic theory is a theory of chemical combination, first stated by John Dalton in 1803. It involves the following postulates:
1. Elements consist of indivisible small particles (atoms).
2. All atoms of the same element are identical; different elements have different types of atom.
3. Atoms can neither be created nor destroyed.
4. ’Compound elements’ (i.e. compounds) are formed when atoms of different elements join in simple ratios to form ’compound atoms’ (i.e. molecules).
Dalton also proposed symbols for atoms of different elements (later replaced by the present notation using letters).
darmstadtium → darmstadtij
Darmstadtium was discovered by S. Hofmann et al. collaboration at the Heavy Ion Research Laboratory (Gesellschaft für Schwerionenforschung, GSI) in Darmstadt, Germany in November 1994. The title honours the Laboratory for Heavy Ion Research (called GSI) in Darmstadt, Germany, where the substance was first made. It is synthetic radioactive metal. The fusion-evaporation reaction using a 62Ni beam on an isotopically enriched 208Pb target produced four chains of alpha-emitting nuclides following the presumed formation of 269110 + 1n.
Lewis, Gilbert N. → Lewis, Gilbert N.
Gilbert Newton Lewis (1875-1946) is an American chemist whose theory of the electron pair fostered understanding of the covalent bond and extended the concept of acids and bases.
ligand field theory → teorija ligandnog polja
Ligand field theory is a description of the structure of crystals containing a transition metal ion surrounded by nonmetallic ions (ligands). It is based on the construction of molecular orbitals involving the d-orbitals of the central metal ion and combinations of atomic orbitals of the ligands.
Citing this page:
Generalic, Eni. "PloÅ¡no centrirana kubiÄna reÅ¡etka." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table