nuclear magnetic resonance → nuklearna magnetska rezonancija
Nuclear magnetic resonance (NMR) is a type of radio-frequency spectroscopy based on the magnetic field generated by the spinning of electrically charged atomic nuclei. This nuclear magnetic field is caused to interact with a very large (1 T - 5 T) magnetic field of the instrument magnet. NMR techniques have been applied to studies of electron densities and chemical bonding and have become a fundamental research tool for structure determinations in organic chemistry.
magnetic permeability → magnetska permeabilnost
Magnetic permeability (μ), also called permeability, is a constant of proportionality that exists between magnetic induction and magnetic field intensity. This constant is equal to approximately μo = 1.257×10-6 H/m in a vacuum.
Magnetic permeability is often expressed in relative, rather than in absolute, terms. If μ represents the permeability of the substance in question, then the relative permeability, μr, is given by:
resonance → rezonancija
Resonance is a stabilising quality of certain molecules that can be represented by considering the electron distribution in an ion or molecule as a composite of two or more forms, in those cases where a single form is an inadequate representation; for example, benzene and the carbonate ion. A various canonical structures can be drawn to show how electron delocalisation will explain the discrepancy, the difference in electron density
nuclear reactor → nuklearni reaktor
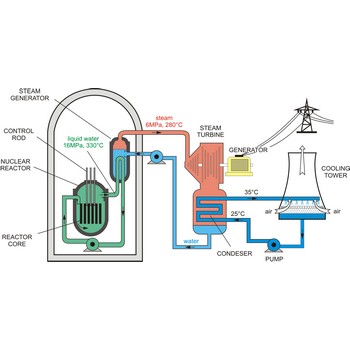
Nuclear reactor is an assembly of fissionable material (uranium-235 or plutonium-239) designed to produce a sustained and controllable chain reaction for the generation of electric power.
The essential components of a nuclear reactor are:
- The core, metal rods containing enough fissionable material to maintain a chain reaction at the necessary power level (as much as 50 t of uranium may be required).
- A source of neutrons to initiate the reaction (such as a mixture of polonium and beryllium)
- A moderator to reduce the energy of fast neutrons for more efficient fission (material such as graphite, beryllium, heavy water, and light water are used)
- A coolant to remove the fission-generated heat (water, sodium, helium, and nitrogen may be used)
- A control system such as rods of boron or cadmium that have high capture cross sections (to absorb neutrons)
- Adequate shielding, remote-control equipment, and appropriate instrumentation are essential for personnel safety and efficient operation.
spin → spin
Spin is the intrinsic angular momentum of an elementary particle, or system of particles such as nucleus, that is also responsible for the magnetic moment; or, a particle or nucleus possessing such a spin. The spins of nuclei have characteristic fixed values. Pairs of neutrons and protons align to cancel out their spins, so that nuclei with an odd number of neutrons and/or protons will have a net non-zero rotational component characterized by a non-zero quantum nuclear spin number.
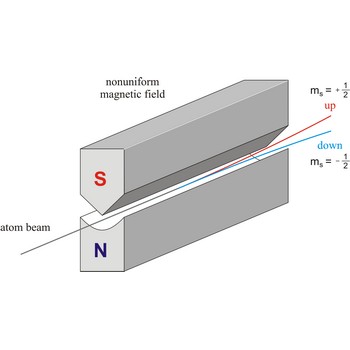
Stern-Gerlach experiment: a beam of silver atoms is split into two beams when it traverses a nonuniform magnetic field. Atoms with spin quantum number ms=+1/2 follow one trajectory, and those with ms=+1/2 follow another.
actinides → aktinoidi
Actinides (actinons or actinoids) are the fourteen elements from thorium to lawrencium inclusive, which follow actinium in the periodic table. The position of actinium is somewhat equivocal and, although not itself an actinide, it is often included with them for comparative purpose. The series includes the following elements: thorium (Th), protactinium (Pa), uranium (U), neptunium (Np), plutonium (Pu), amercium (Am), curium (Cm), berkelium (Bk), californium (Cf), einsteinium (Es), fermium (Fm), mendelevium (Md), nobelium (No) and lawrencium (Lr). Every known isotope of the actinide elements is radioactive. Traces of Pa, Np and Pu are consequently found, but only Th and U occur naturally to any useful extent.
americium → americij
Americium was discovered by Glenn T. Seaborg, Ralph A. James, Stanley G. Thompson and Albert Ghiorso (USA) in 1944. Named for the American continent. It is silvery-white, artificially produced radioactive metal. Americium was produced by bombarding plutonium with neutrons. Americium-241 is currently used in smoke detectors.
artificial radioactive isotope → umjetni radioaktivni izotop
Artificial radioactive isotopes are formed when an atom is bombed with an accelerator or exposing it to slow moving neutrons in a nuclear reactor. In this way isotopes (radionuclides) are obtained which are non-existent in nature because of their unstability and radioactive transition into stable isotopes. Most important radioactive isotopes are:
Radioactive isotope of cobalt is formed when ordinary metal cobalt is bombed with neutrons in a nuclear reactor.
Radioactive isotope of phosphorus is formed when ordinary phosphorus is bombed with deuterons produced in cyclotron.
radioactive isotope of carbon is formed when a nitrogen is bombed with slow moving neutrons in a nuclear reactor. It is mostly used as a radioactive indicator.
artificial radioactivity → umjetna radioaktivnost
Artificial radioactivity is a creation, with the help of an accelerator or in the nuclear reactor, of isotopes (radionuclides) which are found in nature because they are unstable and by radioactive conversion they are converted to stable isotopes.
Citing this page:
Generalic, Eni. "Nuklearna magnetska rezonancija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table