stainless steel → nehrđajući čelik
Stainless steel is an iron alloy that contains at least 10.5 % chromium and carbon (under 1.5 %).
unit cell → jedinična ćelija
Unit cell is the smallest fragment of the structure of a solid that by repetition can generate the entire structure.
electrolytic cell → elektrolitska ćelija
Electrolytic cell is an electrochemical cell that converts electrical energy into chemical energy. The chemical reactions do not occur spontaneously at the electrodes when they are connected through an external circuit. The reaction must be forced by applying an external electric current. It is used to store electrical energy in chemical form (rechargeable battery). It is also used to decompose or produce (synthesise) new chemicals by the application of electrical power. This process is called electrolysis, e.g., water can be decomposed into hydrogen gas and oxygen gas. The free energy change of the overall cell reaction is positive.
Gratzel solar cell → Gratzelova sunčeva ćelija
Grätzel solar cell is photoelectrochemical cell, developed by Michael Grätzel and collaborators, simulates some characteristics of the natural solar cell, which enables photosynthesis take place. In natural solar cell the chlorophyll molecules absorb light (most strongly in the red and blue parts of the spectrum, leaving the green light to be reflected). The absorbed energy is sufficient to knock an electron from the excited chlorophyll. In the further transport of electron, other molecules are involved, which take the electron away from chlorophyll. In Grätzel cell, the tasks of charge-carrier generation and transport are also assigned to different species.
His device consists of an array of nanometre-sized crystallites of the semiconductor titanium dioxide, welded together and coated with light-sensitive molecules that can transfer electrons to the semiconductor particles when they absorb photons. So, light-sensitive molecules play a role equivalent to chlorophyll in photosynthesis. In Grätzel cell, the light-sensitive molecule is a ruthenium ion bound to organic bipyridine molecules, which absorb light strongly in the visible range; titanium dioxide nanocrystals carry the received photoexcited electrons away from electron donors. On the other hand, a donor molecule must get back an electron, so that it can absorb another photon. So, this assembly is immersed in a liquid electrolyte containing molecular species (dissolved iodine molecules) that can pick up an electron from an electrode immersed in the solution and ferry it to the donor molecule. These cells can convert sunlight with efficiency of 10 % in direct sunlight and they are even more efficient in diffuse daylight.
solar cell → sunčeva ćelija
Solar cell, or photovoltaic cell, is a device that captures sunlight and transforms it directly to electricity. All solar cells make use of photovoltaic effect, so often they are called photovoltaic cells. Almost all solar cells are built from solid-state semiconducting materials, and in the vast majority of these the semiconductor is silicon.
The photovoltaic effect involves the generation of mobile charge carriers-electrons and positively charged holes-by the absorption of a photon of light. This pair of charge carriers is produced when an electron in the highest filled electronic band of a semiconductor (the valence band) absorbs a photon of sufficient energy to promote it into the empty energy band (the conduction band). The excitation process can be induced only by a photon with an energy corresponding to the width of the energy gap that separates the valence and the conduction band. The creation of an electron-hole pair can be converted into the generation of an electrical current in a semiconductor junction device, wherein a layer of semiconducting material lies back to back with a layer of either a different semiconductor or a metal. In most photovoltaic cells, the junction is p-n junction, in which p-doped and n-doped semiconductors are married together. At the interface of the two, the predominance of positively charged carriers (holes) in the p-doped material and of negatively charged carriers (electrons) in the n-doped material sets up an electric field, which falls off to either side of the junction across a space-charge region. When absorption of a photon in this region generates an electron-hole pair, these charge carriers are driven in opposite directions by the electric field, i.e. away from the interface and toward the top and bottom of the two-layer structure, where metal electrodes on these faces collect the current. The electrode on the top layer (through which light is absorbed) is divided into strips so as not to obscure the semiconducting layers below. In most widely used commercial solar cells, the p-doped and n-doped semiconductive layers are formed within a monolithic piece of crystalline silicon. Silicon is able to absorb sunlight at those wavelengths at which it is most intense-from the near-infrared region (wavelengths of around 1200 nm) to the violet (around 350 nm).
chromium → krom
Chromium was discovered by Louis-Nicholas Vauquelin (France) in 1797. The origin of the name comes from the Greek word chroma meaning colour. It is very hard, crystalline, steel-grey metal. The pure metal has a blue-white colour. It is hard, brittle and corrosion-resistant at normal temperatures. Hexavalent compounds toxic by skin contact. The most important chromium mineral is chromite [Fe,Mg(CrO4)]. Produced commercially by heating its ore in the presence of silicon or aluminium. Used to make stainless steel. It gives the colour to rubies and emeralds. Iron-nickel-chromium alloys in various percentages yield an incredible variety of the most important metals in modern technology.
lutetium → lutecij
Lutetium was discovered by Georges Urbain (France) and independently by Carl Auer von Welsbach (Austria) in 1907. The origin of the name comes from the Greek word Lutetia meaning Paris. It is silvery-white and relatively stable in air, rare earth metal. Lutetium is found with ytterbium in gadolinite and xenotime. Stable lutetium nuclides can be used as catalysts in cracking, alkylation, hydrogenation, and polymerization.
ytterbium → iterbij
Ytterbium was discovered by Jean de Marignac (France) in 1878. Named after Ytterby, a village in Sweden. It is silvery, lustrous, malleable and ductile metal. Oxidizes slowly in air. Reacts with water. Flammable dust. Ytterbium is found in minerals such as yttria, monazite, gadolinite and xenotime. Used in metallurgical and chemical experiments.
amperometry → amperometrija
Amperometry is determining the concentration of a material in a sample by measuring electric current passing through a cell containing the solution.
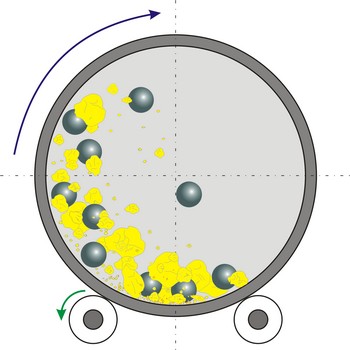
ball mill → kuglični mlin
Ball mill is a grinder for reducing hard materials to powder. The grinding is carried out by the pounding and rolling of a charge of steel or ceramic balls carried within the cylinder. The cylinder rotates at a relatively slow speed, allowing the balls to cascade through the mill base, thus grinding or dispersing the materials.
Type of ball mills, centrifugal and planetary mills, are devices used to rapidly grind materials to colloidal fineness (approximately 1 μm and below) by developing high grinding energy via centrifugal and/or planetary action.
Citing this page:
Generalic, Eni. "Nehrđajući čelik." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table