phase diagram → fazni dijagram
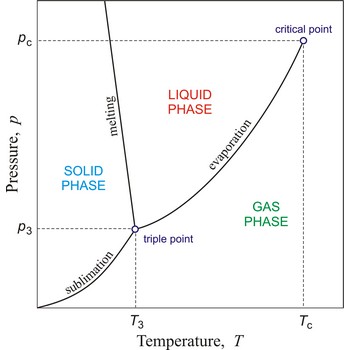
Phase diagram is a graphic representation of the equilibrium relationships between phases (such as vapour-liquid, liquid-solid) of a chemical compound, mixture of compounds, or solution.
The figure shows a typical phase diagram of an element or a simple compound. The stability of solid, liquid and gas phases depends on the temperature and the pressure. The three phases are in equilibrium at the triple point. The gas and liquid phases are separated by a phase transition only below the temperature of the critical point.
reflux condenser → povratno hladilo
Reflux condenser is used for repeated transformation of vapour in liquid in order to prevent the loss due to evaporation.
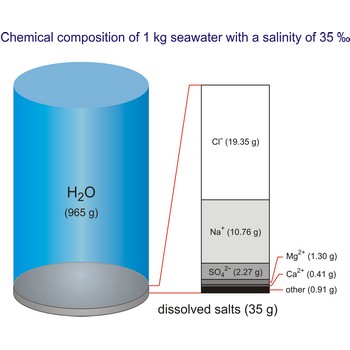
salt water → slana voda
Salt water is the water of the sea and the ocean. This water contains a relatively high percentage of dissolved salt (about 35 g of salt per 1 000 g of sea water.). About 90 % of that salt would be sodium chloride, or ordinary table salt.
The salinity of ocean water varies. It is affected by such factors as melting of ice, inflow of river water, evaporation, rain, etc.
solution composition → sastav otopine
Solutions are homogenous mixtures of several components. The component which is found in a greater quantity is called the solvent and the other components are called solutes. Quantitative composition of a solution can be expressed by concentration (amount, mass, volume and number), by fraction (amount, mass, and volume), ratio (amount, mass, and volume) and by molality. Amount, mass, and volume ratio are numerical, nondimensional units and are frequently expressed as percentage (% = 1/100), promile (‰ = 1/1000) or parts per million (ppm = 1/1 000 000). If it is not defined, it is always related to the mass ratio.
Soxhlet extractor → Soxhletov ekstraktor
Soxhlet extractor is a laboratory apparatus designed to extract substances with a low solubility in the extracting solvent. The method described by the German chemist Franz von Soxhlet (1848-1926) in 1879 is the most commonly used example of a semi-continuous method applied to extraction of lipids from foods. In the Soxhlet extractor, the sample soaks in hot solvent that is periodically siphoned off, distilled and returned to the sample. During each cycle, a portion of the non-volatile compound dissolves in the solvent. After many cycles the desired compound is concentrated in the distillation flask. The solvent in the flask is then evaporated and the mass of the remaining lipid is measured.
spectrophotometer → spektrofotometar
Spectrophotometer is an instrument for measuring the amount of light absorbed by a sample.
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
van der Waals’ equation → van der Waalsova jednadžba
Van der Waals’ equation is an equation of state for real fluids which takes the form:
where p is pressure, Vm is molar volume, T is temperature, R is the molar gas constant, and a and b are characteristic parameters of the substance which describe the effect of attractive and repulsive intermolecular forces.
Citing this page:
Generalic, Eni. "Molarna entalpija isparavanja." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table