chemisorption → kemisorpcija
Chemisorption is a binding of a liquid or gas on the surface or in the interior of a solid by chemical bonds or forces.
atom radius → radijus atoma
Atoms and molecules have no strict boundaries. The volume of a free atom is usually defined as that volume that contains 90 % of electron cloud. The radius of an atom represents half of interatom distance of two identical atoms which are in touch but are not interconnected either by a covalent or an ionic bond, but with a very weak van der Waals’s bond.
beta-glucan → beta-glukan
Beta-glucans are are naturally occurring polysaccharides that contain only glucose as structural components, and are linked with β-glycosidic bonds. They is the most known powerful immune stimulant. The most active forms of β-glucans are those comprising D-glucose units with β(1→3) links and with short side-chains of D-glucose attached at the β(1→6) position. These are referred to as beta-1,3/1,6 glucan. They are a major component of soluble dietary fiber, which can be found in cereal grains (oats, barley, wheat), yeast, and certain mushrooms (shiitake, maitake).
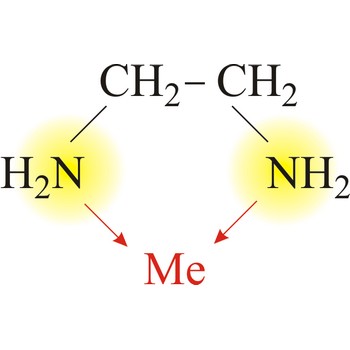
bidentate ligand → bidentatni ligand
Bidentate ligand is a ligand that has two "teeth" or atoms that coordinate directly to the central atom in a complex. An example of a bidentate ligand is ethylenediamine. A single molecule of ethylenediamine can form two bonds to a metal ion. The bonds form between the metal ion and the nitrogen atoms of ethylenediamine.
borane → borani
Borane is any of the group of compounds of boron and hydrogen (B2H6, B4H10, B5H9, B5H11...), many of which can be prepared by action of acid on magnesium boride (Mg3B2). Boranes are a remarkable group of compounds in that their structures cannot be described using the conventional two-electron covalent bond model.
branched chain → razgranati lanac
Branched chain is an open chain of atoms with one or more side chains attached to it.
carbon → ugljik
Carbon has been known since ancient times. The origin of the name comes from the Latin word carbo meaning charcoal. Graphite form of carbon is a black, odourless, slippery solid. Graphite sublimes at 3825 °C. Diamond form is a clear or colored; an extremely hard solid. C60 is Buckminsterfullerine. Carbon black burns readily with oxidants. Carbon is made by burning organic compounds with insufficient oxygen. There are close to ten million known carbon compounds, many thousands of which are vital to organic and life processes. Radiocarbon dating uses the carbon-14 isotope to date old objects.
covalent compound → kovalentni spoj
Covalent compound is a compound made of molecules - not ions, such as H2O, CH4, Cl2. The atoms in the compound are bound together by shared electrons. Also called a molecular compound.
Citing this page:
Generalic, Eni. "Konjugirana dvostruka veza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table