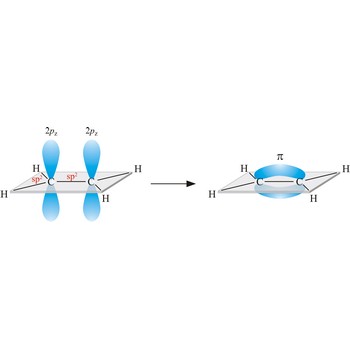
conjugated double bond → konjugirana dvostruka veza
Conjugated double bond in organic compounds is a system of double bonds between atoms which are separated by one single bond (1,3-butene, H2C=CH-CH=CH2).
conjugated bond → konjugirana veza
Conjugated bonds describe the alternating pattern of double and single bonds, or triple bonds and single bonds, in a molecule. In such molecules, there is some delocalisation of electrons into the pi orbitals of the carbon atoms linked by the single bond.
cumulated double bond → kumulirana dvostruka veza
Cumulated double bond in organic compounds is a system of two double bonds on the same atom of carbon (C=C=C)
hydrogen bond → vodikova veza
Hydrogen is a bond formed by a hydrogen atom to an electronegative atom, and is denoted by dashed lines H-X---H-B. A hydrogen atom covalently bound to an oxygen (electronegative atom) has a significant positive charge and can form a weak bond to another electronegative atom.
conjugated acid → konjugirana kiselina
Conjugated acid is a particle that develops after a base receives a proton.
conjugated base → konjugirana baza
Conjugated base is a particle which is left after a molecule of acid releases a proton.
covalent bond → kovalentna veza
Covalent bond is a chemical bond between two atoms whose stability results from the sharing of two electrons, one from each atom (H· + ·H = H:H or H-H).
metallic bond → metalna veza
Metallic bond is a electrostatic attraction binding the positive ions of a solid metal together by means of a "sea" of delocalised valence electrons
polar covalent bond → polarna kovalentna veza
Polar covalent bond is a covalent bond in which the electrons are not equally shared because one atom attracts them more strongly than the other
Citing this page:
Generalic, Eni. "Konjugirana dvostruka veza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table