dissociation constant → konstanta disocijacije
Dissociation constant is a constant whose numerical value depends on the equilibrium between the undissociated and dissociated forms of a molecule. A higher value indicates greater dissociation.
The term dissociation is also applied to ionisation reactions of acids and bases in water. For example
which is often regarded as a straightforward dissociation into ions
The equilibrium constant of such a dissociation is called the acid dissociation constant or acidity constant, given by
The concentration of water [H2O] can be taken as constant.
Similarly, for a base, the equilibrium
is also a dissociation; with the base dissociation constant or basicity constant, given by
Ka (Kb) is a measure of the strength of the acid (base).
histidine → histidin
Histidine is an electrically charged amino acids with basic side chains. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Histidine is perhaps the most common and versatile catalytic residue in proteins. The imidazole sidechain of histidine has a pKa of approximately 6.0. This means that, at physiologically relevant pH values, relatively small shifts in pH will change its average charge. The unprotonated imidazole is nucleophilic and can serve as a general base, while the protonated form can serve as a general acid. In addition, it is often a ligand for transition metal ions such as iron and zinc.
- Abbreviations: His, H
- IUPAC name: 2-amino-3-(1H-imidazol-5-yl)propanoic acid
- Molecular formula: C6H9N3O2
- Molecular weight: 155.15 g/mol
indicator → indikator
Indicator is a substance used to show the presence of a chemical substance or ion by its colour. Acid-base indicators are compounds, such as phenolphtaleine and methyl orange, which change colour reversibly, depending on whether the solution is acidic or basic. Oxidation-reduction indicators are substances that show a reversible colour change between oxidised and reduced forms.
lithosphere → litosfera
Lithosphere (from the Greek for rocky sphere) is rigid, rocky outer layer of the Earth, consisting of the crust and the solid outermost layer of the upper mantle. The distinguishing characteristic of the lithosphere is not its composition but its flow properties. It floats on the asthenosphere, which is the heat-softened layer of the mantle below the lithosphere.
The lithospheric is not one continuous piece but is broken into about a dozen major separate rigid blocks, or plates, which move independently relative to one another. This movement of lithospheric plates over the asthenosphere is described as plate tectonics. When an oceanic plate and a continental plate meet, the heavier oceanic plate (composed mostly of basalt, specific gravity about 3.0 or peridotite, specific gravity about 3.3) subducts under the lighter continental plate (composed mostly of granite, specific gravity about 2.7).
living planet index → indeks života na planetu
The Living Planet Index (LPI) reflects changes in the health of the planet's ecosystems by tracking trends in nearly 8000 populations of vertebrate species. The LPI first calculates the annual rate of change for each species population in the dataset, then calculates the average change across all populations for each year from 1970, when data collection began, to 2007, the latest date for which data is available.
The Global LPI shows a decline of around 30 % from 1970 to 2007, based on 7953 populations of 2544 species of birds, mammals, amphibians, reptiles and fish.
lysine → lizin
Lysine is an electrically charged amino acids with basic side chains. Lysine is a base, as are arginine and histidine. The amino group is highly reactive and often participates in reactions at the active centers of enzymes. Lysine plays an important role in coordinating negatively charged ligands. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Lys, K
- IUPAC name: 2,6-diaminohexanoic acid
- Molecular formula: C6H14N2O2
- Molecular weight: 146.19 g/mol
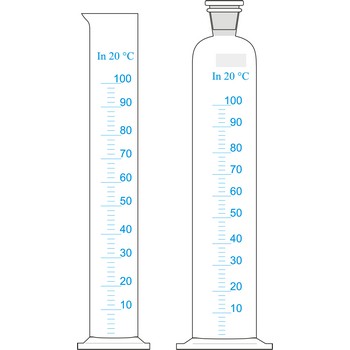
measuring cylinder → menzura
Measuring cylinders (graduated cylinders) are graduated glass cylinders with a capacity from 2 mL to 2 L. They are used when reagent solutions for volume measuring are prepared when accuracy is not of great relevance. The larger the measuring cylinder, the bigger the measuring error.
methyl orange → metil oranž
Methyl orange is an acid-base indicator, in acid it turns red and in a base it turns yellow.
nucleic acid → nukleinska kiselina
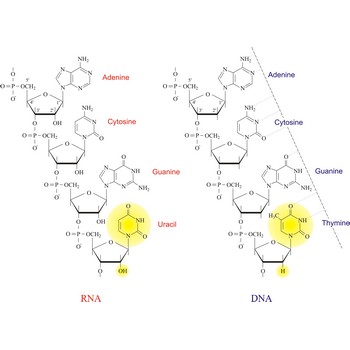
Nucleic acids are a complex, high-molecular-weight biochemical macromolecules composed of nucleotide chains that convey genetic information. The most common nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Each nucleic acid chain is composed of subunits called nucleotides, each containing a sugar, a phosphate group, and nitrogenous base. DNA was first discovered in 1869 by the Swiss biochemist Friedrich Miescher (1844-1895).
Both DNA and RNA contain the two major purine bases adenine (A) and guanine (G) and one of the major pyrimidines, cytosine (C). Of the other two pyrimidines, thymine (T) is found in DNA and uracil (U) is found in RNA. There are two major pentoses in nucleic acids:2'-deoxy-D-ribose in DNA and D-ribose in RNA.
Nucleotides are linked together in both DNA and RNA in a polymeric fashion via covalent bonds. These bonds exist through phosphate-group bridges in which the 5' hydroxyl group of one nucleotide unit is joined to the 3' hydroxyl group of the next nucleotide. RNA is usually a single-stranded molecule, whereas DNA is usually double-stranded.
Citing this page:
Generalic, Eni. "Konjugirana baza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table