saturated fatty acid → zasićena masna kiselina
Saturated fatty acid is a fatty acid carrying the maximum possible number of hydrogen atoms (It doesn’t have any double bounds in the alkyl chain). The most important of these are:
| Butyric (butanoic acid) | CH3(CH2)2COOH |
| Lauric (dodecanoic acid) | CH3(CH2)10COOH |
| Myristic (tetradecanoic acid) | CH3(CH2)12COOH |
| Palmitic (hexadecanoic acid) | CH3(CH2)14COOH |
| Stearic (octadecanoic acid) | CH3(CH2)16COOH |
| Arachidic (eicosanoic acid) | CH3(CH2)18COOH |
unsaturated fatty acid → nezasićena masna kiselina
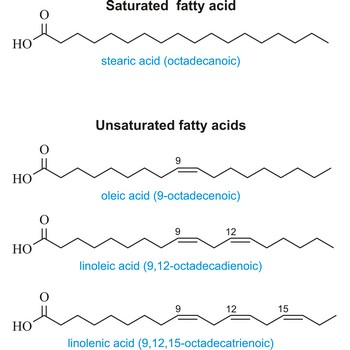
Unsaturated fatty acid is a fatty acid whose carbon chain can absorb additional hydrogen atoms. Their carbon chain has one or more double or triple valence bond per molecule. The most important of these are:
| Oleic (9-octadecenoic acid) | CH3(CH2)7CH=CH(CH2)7COOH |
| Linoleic (9,12-octadecadienoic acid) | CH3(CHCH2)3(CH2CH=CH)2(CHCH2)7COOH |
| Linolenic (9,12,15-octadecatrienoic acid) | CH3(CH2CH=CH)3(CHCH2)7COOH |
oxygen → kisik
Oxygen was discovered by Joseph Priestley (England) in 1774. The origin of the name comes from the Greek words oxy genes meaning acid and forming (acid former). It is colourless, odourless gas; pale blue liquid. Extremely reactive. Forms oxides with nearly all other elements except noble gases. It is the most abundant element in the earth’s crust and makes up almost 21 % of the atmosphere. Oxygen is obtained primarily from liquid air by fractional distillation. Small amounts are made in the laboratory by electrolysis of water. Used in steel making, welding and supporting life. Naturally occurring ozone (O3) in the upper atmosphere shields the earth from ultraviolet radiation.
acetal → acetal
Acetals are organic compounds having the structure R2C(OR’)2 (R’ ≠ H). They are organic compounds formed by addition of alcohol molecules to aldehyde or ketone molecules. Originally, the term was confined to derivatives of aldehydes (one R = H), but it now applies equally to derivatives of ketones (neither R = H ). Mixed acetals have different R’ groups. The formation of acetals is reversible; acetals can be hydrolysed back to aldehydes (ketone) in acidic solutions.
Acetal, 1,1-diethoxyethane (CH3CH(OC2H5)2), is an organic compound, pleasant smelling, formed by addition of ethyl alcohol to ethanal (acetaldehyde). It is used as a solvent and in synthetic organic chemistry.
alkalinity → alkalitet
Alkalinity is a measure of a material’s ability to neutralize acids. It is usually determined using titration.
americium → americij
Americium was discovered by Glenn T. Seaborg, Ralph A. James, Stanley G. Thompson and Albert Ghiorso (USA) in 1944. Named for the American continent. It is silvery-white, artificially produced radioactive metal. Americium was produced by bombarding plutonium with neutrons. Americium-241 is currently used in smoke detectors.
amino acids → aminokiseline
Amino acids are compounds containing both a carboxylic acid group (-COOH) and an amino group (-NH2 ). The most important are the α-amino acids, in which the -NH2 group in attached to the C atom adjacent to the -COOH group. In the β-amino acids, there is an intervening carbon atom.
acylaction reaction → reakcije aciliranja
Acylaction reaction involves the introduction of an acyl group (RCO-) into a compound. An alkyl halide is reacted with an alcohol or a carboxylic acid anhydride e.g.
The introduction of an acetyl group (CH3CO-) is acetylation, a process used for protecting -OH groups in organic synthesis.
Citing this page:
Generalic, Eni. "Kisela kiša." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table