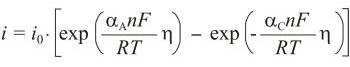battery → baterija
Battery a device that converts chemical energy to electrical energy. The process underlying the operation of a battery involves a chemical reaction in which electrons are transferred from one chemical species to another. This process is carried out in two half-reactions, one that involves the loss of electrons and one that involves their gain. The battery is an electrochemical cell divided in two half-cells, and reaction proceeds when these are connected together by an electrically conducting pathway. The passage of electrons from one half-cell to the other corresponds to an electric current. Each half-cell contains an electrode in contact with the reacting species. The electrode which passes electrons into the circuit when battery discharges is called anode and is negative terminal. The electrode which receives electrons is called cathode, and is the battery’s positive terminal. The electrical circuit is completed by an electrolyte, an electrically conducting substance placed between the two electrodes which carriers a flow of charge between them. In wet cells, the electrolyte is a liquid containing dissolved ions, whose motion generates an electrical current; in dry cells the electrolyte is basely solid, for example, a solid with mobile ions or porous solid saturated with an ionic solution.
Bunsen’s cell → Bunsenov članak
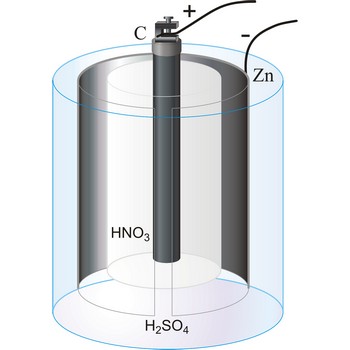
Bunsen’s cell is a primary cell devised by Robert W. Bunsen consisting of a zinc cathode immersed in dilute sulphuric acid and carbon anode immersed in concentrated nitric acid. The electrolytes are separated by a porous pot. The cell gives an e.m.f. of about 1.9 V.
Butler-Volmer equation → Butler-Volmerova jednadžba
Butler-Volmer equation is an activation controlled reaction, the one for which the rate of reaction is controlled solely by the rate of the electrochemical charge transfer process, which is in turn an activation-controlled process. This gives rise to kinetics that are described by the Butler-Volmer equation:
where io is exchange current density, η is overpotential (η = E - Eo), n is number of electrons, αA is anodic transfer coefficient, and αC is cathodic transfer coefficient
coal gas → ugljeni plin
Coal gas is a gas produced by the destructive distillation of coal, and contains approximately 50 % hydrogen, 35 % methane and 8 % carbon monoxide. The by-products of the production of coal gas are coal tar and coke.
epimerization → epimerizacija
Epimerization is an interconversion of epimers. If the conversion of one epimer to another is catalyzed by an enzyme, the enzyme is an epimerase.
fermentation → fermentacija
Fermentation is a class of biochemical reactions that break down complex organic molecules (such as carbohydrates) into simpler materials (such as ethanol, carbon dioxide, and water). Fermentation reactions are catalyzed by enzymes.
formaldehyde → formaldehid
Formaldehyde (methanal) is a colourless gas, HCHO; r.d. 0.815 (at -20 °C); m.p. -92 °C; b.p. -21 °C. It is the simplest aldehyde, made by the catalytic oxidation of methanol (500 °C; silver catalyst) by air. It forms two polymers: methanal trimer and polymethanal.
groundbed → zemljana anoda
Groundbed is a buried item, such as junk steel or graphite rods, that serves as the anode for the cathodic protection of pipelines or other buried structures.
cell potential → potencijal članka
Cell potential (E) is difference between anode and cathode potential. If the cell potential is positive, then the reaction is spontaneous.
Citing this page:
Generalic, Eni. "Kate greenaway." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table