charge number → nabojni broj
Charge number represents ion charge of an atom or a group. It is denoted as a right superscript (Fe2+ or SO42-).
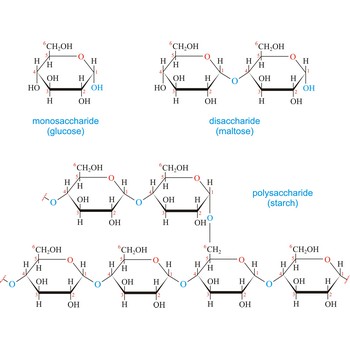
carbohydrate → ugljikohidrat
Carbohydrates (often called carbs for short) are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis. They are also known as saccharides, a term derived from the Latin word saccharum for sugar. Carbohydrates are the most abundant class of compounds in the biological world, making up more than 50 % of the dry weight of the Earth’s biomass. Every type of food we eat can have its energy traced back to a plant. Plants use carbon dioxide and water to make glucose, a simple sugar, in photosynthesis. Other carbohydrates such as cellulose and starch are made from the glucose. Light from the sun is absorbed by chlorophyll and this is converted to the energy necessary to biosynthesize carbohydrates
The term carbohydrate was applied originally to monosaccharides, in recognition of the fact that their empirical composition can be expressed as Cx(H2O)y. Later structural studies revealed that these compounds were not hydrates but the term carbohydrate persists.
Carbohydrates are generally classed as either simple or complex. Simple sugars, or monosaccharides, are carbohydrates that can’t be converted into smaller subunits by hydrolysis. Complex carbohydrates are made of two (disaccharides) or more (oligosaccharides, polysaccharides) simple sugars linked together by acetal (glycosidic) bonds and can be split into the former by hydrolysis.
conjugated protein → konjugirani protein
Conjugated proteins are proteins which have a prostetic group as a part of their structure which is bonded with one or more amino acids of the same protein.
derivative → derivat
Derivative is a compound that is derived from some other compound and usually maintains its general structure, e.g. trichlormethane (chloroform) is a derivative of methane.
diazo compounds → diazo-spojevi
Diazo compounds are compounds having the divalent diazo group, =N+=N-, attached to a carbon atom. The term includes azo compounds, diazonium compounds, and also such compounds as diazomethane, CH2=N2.
ionisation energy → energija ionizacije
Ionisation energy is the minimum energy required to remove an electron from an isolated atom or molecule (in its vibrational ground state) in the gaseous phase.
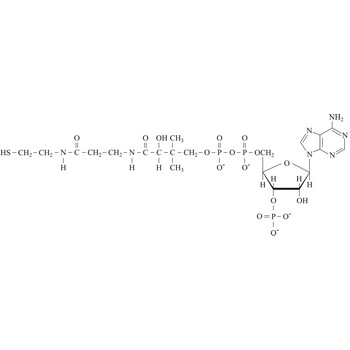
coenzyme a → koenzim a
Coenzyme A (CoA) is an essential metabolic cofactor synthesized from cysteine, pantothenate (vitamin B5), and ATP. CoA plays important roles in many metabolic pathways, including the tricarboxylic acid (TCA) cycle, and the synthesis and oxidation of fatty acids. One of the main functions of CoA is the carrying and transfer of acyl groups. Acylated derivatives (acetyl-CoA) are critical intermediates in many metabolic reactions.
coenzyme q → koenzim q
Coenzyme Q (CoQ) or ubiquinone is any of a group of related quinone-derived compounds that serve as electron carriers in the electron transport chain reactions of cellular respiration. There are some differences in the length of the isoprene unit (in bracket on left) side chain in various species. All the natural forms of CoQ are insoluble in water, but soluble in membrane lipids.
concentration → koncentracija
1. Group of four quantities characterizing the composition of a mixture with respect to the volume of the mixture (mass, amount, volume and number concentration).
2. Short form for amount (of substance) concentration (substance concentration in clinical chemistry).
Citing this page:
Generalic, Eni. "Karbonilna skupina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table