sucrose → saharoza
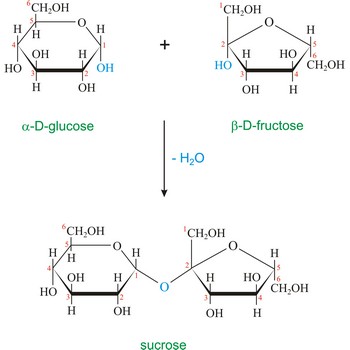
Sucrose (saccharose), or ordinary table sugar, is a disaccharide in which α-D-glucopyranose and β-D-fructofuranose are joined at their anomeric carbons by a glycosidic bond. There are no hemiacetals remaining in the sucrose and therefore sucrose is not a reducing sugar and does not exhibit mutarotation. Sugar is a white crystalline sweet compound found in many plants and extracted from sugar cane and sugar beet. It is used as a sweetening agent in food and drinks. If heated to 200 °C, sucrose becomes caramel. When sucrose is hydrolyzed it forms an equimolar mixture of glucose and fructose. This mixture of monosaccharides is called invert sugar. Honeybees have enzymes called invertases that catalyze the hydrolysis of sucrose. Honey, in fact, is primarily a mixture of glucose, fructose, and sucrose.
sugar → šećer
Sugar is any of a group of water-soluble carbohydrates of relatively low molecular weight and typically having a sweet taste. The group comprises mainly monosaccharides (glucose, fructose, galactose), disaccharides (sucrose, lactose, maltose), and trisaccharides (raffinose). Many monosaccharides and disaccharides fairly commonly found in nature bear names reflecting the source from which they were first isolated. For example, glucose is also known as grape sugar, lactose as milk sugar, and maltose as malt sugar. In everyday usage, the name is often used to refer specifically to sucrose (table sugar, cane sugar, beet sugar).
supercritical fluid chromatography → superkritična fluidna kromatografija
Supercritical fluid chromatography (SFC) is a hybrid of gas and liquid chromatography. SFC is of importance because it permits the separation and determination of a group of compounds that are not conveniently handled by either gas or liquid chromatography. These compounds are either nonvolatile or thermally labile so that gas chromatography cannot be used and they do not contain functional groups that make possible detection by liquid chromatography. SFC has been applied to a wide variety of materials including natural prodcuts, drugs, foods, pesticides and herbicides, fossil fuels, explosives and propellants.
threonine → treonin
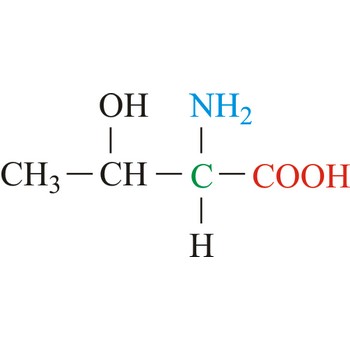
Threonine is neutral amino acids with polar side chains. It differs from serine by having a methyl substituent in place of one of the hydrogens on the β carbon. Threonine is a site of phosphorylation and glycosylation which is important for enzyme regulation and cell signaling. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Thr, T
- IUPAC name: 2-amino-3-hydroxybutanoic acid
- Molecular formula: C4H9NO3
- Molecular weight: 119.12 g/mol
transition metal → prijelazni element
This group of metals is distinguished from other metals not by their physical properties, but by their electronic structure. Transition metals are elements characterized by a partially filled d subshell. The First Transition Series comprises scandium (Sc), titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni) and copper (Cu). The Second and Third Transition Series include the lanthanides and actinides, respectively.
The transition metals are noted for their variability in oxidation state. Thus, manganese has two electrons in its outside shell and five electrons in the next shell down, and exhibits oxidation states of +1, +2, +3, +4, +5, +6, and +7.
They are also characterised by the fact that well into the series, going from left to right, the properties of the succeeding metals do not differ greatly from the preceding ones.
triols → trioli
Trihydric alcohols (i.e. Triols) are organic compounds containing three hydroxyl groups. The simplest trihydric alcohol is 1,2,3-propane-triol, CH2(OH)CH(OH)CH2(OH), which is also known as glycerol (from the Greek glykys meaning sweet) or glycerin. Glycerol is commercially produced by the hydrolysis of fats.
Glycerol is a by-product in the soap industry and is recovered by suitable means.
tyrosine → tirozin
Tyrosine is hydrophobic amino acids with aromatic side chain. Tyrosine is large aromatic residue that is normally found buried in the interior of a protein and is important for protein stability. Tyrosine has special properties since its hydroxyl side chain may function as a powerful nucleophile in an enzyme active site (when ionized) and is a common site for phosphorylation in cell signaling cascades. Tyrosine absorbs ultraviolet radiation and contributes to the absorbance spectra of proteins. It is not essential (or semi-essential) to the human diet, since it is synthesized in the body from other metabolites.
- Abbreviations: Tyr, Y
- IUPAC name: 2-amino-3-(4-hydroxyphenyl)propanoic acid
- Molecular formula: C9H11NO3
- Molecular weight: 181.19 g/mol
valine → valin
Valine is hydrophobic amino acids with aliphatic side chain. It is a member of the branched-chain amino acid family, along with leucine and isoleucine. Valine differs from threonine by replacement of the hydroxyl group with a methyl substituent, but they are of roughly the same shape and volume. The nonpolar hydrophobic amino acids tend to cluster together within proteins, stabilizing protein structure by means of hydrophobic interactions. Valine is an essential amino acid, which means that it cannot be synthesized in the body and must be obtained through dietary sources.
- Abbreviations: Val, V
- IUPAC name: 2-amino-3-methylbutanoic acid
- Molecular formula: C5H11NO2
- Molecular weight: 117.15 g/mol
yttrium → itrij
Yttrium was discovered by Carl Gustaf Mosander (Sweden) in 1843. Named after Ytterby, a village in Sweden. It is silvery, ductile, fairly reactive metal. Exposed surfaces form oxide film. Easily combustible, reacts with oxygen in water to release hydrogen. Yttrium is found in minerals such as monazite, xenotime and yttria. Combined with europium to make red phosphors for colour TV’s. Yttrium oxide and iron oxide combine to form a crystal garnet used in radar.
zwitterion → dipolarni ion
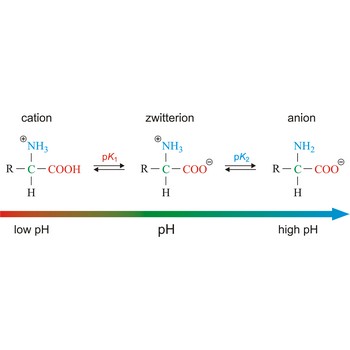
Zwitterion, also known as inner salt or dipolar ion, is an ion with a positive and a negative electrical charge at different locations within a molecule. As the molecule contains two opposite charges, it is electrically neutral. The term zwitterion is derived from the German word zwitter, meaning a hybrid, hermaphrodite. Zwitterions can be formed from compounds that contain both acid groups and base groups in their molecules (ampholytes).
All of the common amino acids found in proteins are ampholytes because they contain a carboxyl group (-COOH) that acts as an acid and an amino group (-NH2) that acts as a base. In the solid state, amino acids exist in the dipolar or zwitterion form. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (H+) ion, and the amino acid becomes positively charged. If base is added, ion removal of the H+ ion from the amino group of the zwitterion produces a negatively charged amino acid.
Citing this page:
Generalic, Eni. "Karbonilna skupina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table