polysaccharide → polisaharid
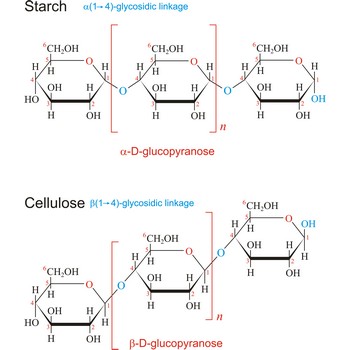
Polysaccharides are compounds consisting of a large number of simple sugars (monosaccharides) linked together by glycosidic bonds. When polysaccharides are composed of a single monosaccharide building block, they are termed homopolysaccharides. Heteropolysaccharides contain two or more different types of monosaccharide. Polysaccharides may have molecular weights of up to several million and are often highly branched. Since they have only the one free anomeric -OH group at the end of a very long chain, polysaccharides aren’t reducing sugars and don’t show noticeable mutarotation. The most common polysaccharides are cellulose, starch, and glycogen.
polystyrene → polistiren
Polystyrene is a vinyl polymer. Structurally, it is a long hydrocarbon chain, with a phenyl group attached to every other carbon atom. Polystyrene is produced by free radical vinyl polymerization, from the monomer styrene. Polystyrene or Styrofoam is used in the construction industry as insulating material and for production of containers.
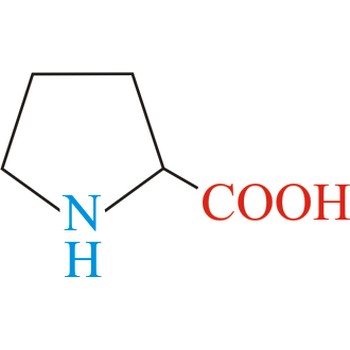
proline → prolin
Proline has an aliphatic side chain with a distinctive cyclic structure. It is unusual because it is conformationally restricted. The secondary amino (imino) group of proline residues is held in a rigid conformation that reduces the structural flexibility of polypeptide regions containing proline. It is not an essential amino acid, which means that the human body can synthesize it.
- Abbreviations: Pro, P
- IUPAC name: pyrrolidine-2-carboxylic acid
- Molecular formula: C5H9NO2
- Molecular weight: 115.13 g/mol
qualitative analysis → kvalitativna analiza
Qualitative analysis involves determining the nature of a pure unknown compound or the compounds present in a mixture. Qualitative inorganic analysis is used to separate and detect cations and anions in a sample substance. According to their properties, cations are usually classified into six groups. Each group has a common reagent which can be used to separate them from the solution.
rhodium → rodij
Rhodium was discovered by William Hyde Wollaston (England) in 1804. The origin of the name comes from the Greek word rhodon meaning rose. It is hard, silvery-white metal. Inert in air and acids. Reacts with fused alkalis. Rhodium is obtained as a by-product of nickel production. Used as a coating to prevent wear on high quality science equipment and with platinum to make thermocouples.
ruthenium → rutenij
Ruthenium was discovered by Karl Karlovich Klaus (Russia) in 1844. The origin of the name comes from the Latin word Ruthenia meaning Russia. It is rare, extremely brittle, silver-grey metal. Unaffected by air, water or acids. Reacts with very hot (molten) alkalis. Ruthenium is found in pentlandite and pyroxinite. Used to harden platinum and palladium. Aircraft magnetos use platinum alloy with 10 % ruthenium.
scandium → skandij
Scandium was discovered by Lars Fredrik Nilson (Sweden) in 1879. The origin of the name comes from the Latin word Scandia meaning Scandinavia. It is fairly soft, silvery-white metal. Burns easily. Tarnishes readily in air. Reaction with water releases hydrogen. Reacts with air and halogens. Scandium occurs mainly in the minerals thortveitile (~34 % scandium) and wiikite. Also in some tin and tungsten ores. Pure scandium is obtained as a by-product of uranium refining. Scandium metal is used in some aerospace applications. Scandium oxide (Sc2O2) is used in the manufacture of high-intensity electric lamps. Scandium iodide (ScI3) is used in lamps that produce light having a colour closely matching natural sunlight.
semiconductor → poluvodič
Semiconductor is a material in which the highest occupied energy band (valence band) is completely filled with electrons at T = 0 K, and the energy gap to the next highest band (conduction band) ranges from 0 to 4 or 5 eV. With increasing temperature electrons are excited into the conduction band, leading to an increase in the electrical conductivity.
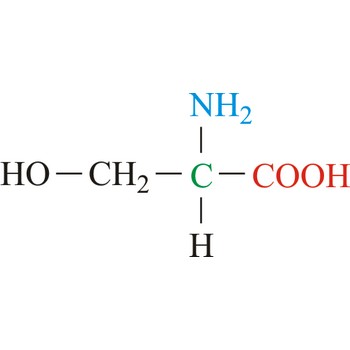
serine → serin
Serine is neutral amino acids with polar side chains. It is one of two hydroxyl amino acids. Both are commonly considered to by hydrophilic due to the hydrogen bonding capacity of the hydroxyl group. Serine often serves as a nucleophile in many enzyme active sites, and is best known for its role in the serine proteases. Serine is a site of phosphorylation and glycosylation which is important for enzyme regulation and cell signaling. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine.
- Abbreviations: Ser, S
- IUPAC name: 2-amino-3-hydroxypropanoic acid
- Molecular formula: C3H7NO3
- Molecular weight: 105.09 g/mol
square pyramidal molecular geometry → kvadratna piramidalna geometrija molekule
Square pyramidal is a molecular shape that results when there are five bonds and one lone pair on the central atom in the molecule. Bromine pentafluoride (BrF5) has the geometry of a square pyramid, with fluorine atoms occupying five vertices, one of which is above the plane of the other four. This molecule is made up of six equally spaced sp3d2 (or d2sp3) hybrid orbitals arranged at 90° angles. The shape of the orbitals is octahedral. Because of the high symmetry of the octahedral arrangement, all six positions are equivalent, so it does not matter in which position in the drawing we put the lone pair. The remaining four atoms connected to the central atom give the molecule a square planar shape.
Citing this page:
Generalic, Eni. "Karbonilna skupina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table