carbonates → karbonati
Carbonates are compounds composed of metal cation and carbonate anion (CO32-), salts of carbonic acid.
Bunsen’s cell → Bunsenov članak
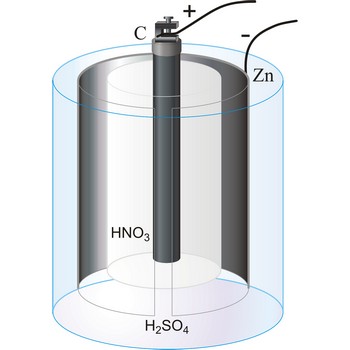
Bunsen’s cell is a primary cell devised by Robert W. Bunsen consisting of a zinc cathode immersed in dilute sulphuric acid and carbon anode immersed in concentrated nitric acid. The electrolytes are separated by a porous pot. The cell gives an e.m.f. of about 1.9 V.
cadmium → kadmij
Cadmium was discovered by Friedrich Strohmeyer (Germany) in 1817. The origin of the name comes from the Latin word cadmia meaning calamine (zinc carbonate, ZnCO3), or from the Greek word kadmeia with the same meaning. It is soft, malleable, blue-white metal. Tarnishes in air, soluble in acids, insoluble in alkalis. Boiling cadmium gives off a weird, yellow-colored vapour that is poisonous. Cadmium can cause a variety of health problems, including kidney failure and high blood pressure. Cadmium is obtained as a by product of zinc refining. The mayor use of cadmium is in electroplating of steel to protect it from corrosion. Also used to make nickel-cadmium batteries. The ability of cadmium to adsorb neutrons has made it of great importance in the design of nuclear reactors. Its compounds are found in paint pigments and a wide variety of intense colours.
cation exchange → kationski izmjenjivač
Cation exchange is a cationic resin has positive ions built into its structure and therefore exchanges negative ions. In the cation exchange, the side groups are ionised acidic groups, such as (-SO3H, -COOH, -OH) to which cations H+ are attached. The exchange reaction is one in which different cations in the solution displace the H+ from the solid.
conjugated base → konjugirana baza
Conjugated base is a particle which is left after a molecule of acid releases a proton.
carbohydrate → ugljikohidrat
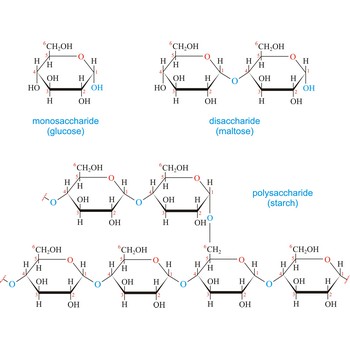
Carbohydrates (often called carbs for short) are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis. They are also known as saccharides, a term derived from the Latin word saccharum for sugar. Carbohydrates are the most abundant class of compounds in the biological world, making up more than 50 % of the dry weight of the Earth’s biomass. Every type of food we eat can have its energy traced back to a plant. Plants use carbon dioxide and water to make glucose, a simple sugar, in photosynthesis. Other carbohydrates such as cellulose and starch are made from the glucose. Light from the sun is absorbed by chlorophyll and this is converted to the energy necessary to biosynthesize carbohydrates
The term carbohydrate was applied originally to monosaccharides, in recognition of the fact that their empirical composition can be expressed as Cx(H2O)y. Later structural studies revealed that these compounds were not hydrates but the term carbohydrate persists.
Carbohydrates are generally classed as either simple or complex. Simple sugars, or monosaccharides, are carbohydrates that can’t be converted into smaller subunits by hydrolysis. Complex carbohydrates are made of two (disaccharides) or more (oligosaccharides, polysaccharides) simple sugars linked together by acetal (glycosidic) bonds and can be split into the former by hydrolysis.
cerium → cerij
Cerium was discovered by Martin Heinrich Klaproth (Germany) and by Jöns Jacob Berzelius (Sweden) in 1803 and Wilhelm von Hisinger (Germany) in 1814. Named after the asteroid Ceres this discovered two years before the element. It is malleable, ductile, iron-grey metal. Tarnishes in air; reacts easily with water. Dissolves in acids; ignites when heated. Metal ignites and burns readily. Strong reductant. Cerium is most abundant rare earth metal. Found in many minerals like monazite sand [Ce(PO4)]. Its oxides are used in the optics and glass-making industries. Its salts are used in the photography and textile industry. Used in high-intensity carbon lamps and as alloying agents in special metals.
Citing this page:
Generalic, Eni. "Karboksilne kiseline." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table