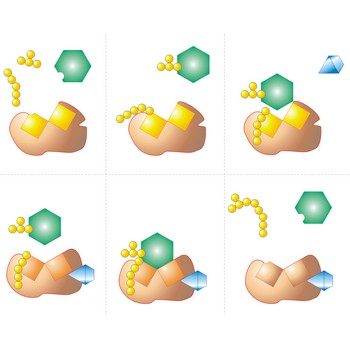
primary alcohol → primarni alkohol
Primary alcohols are alcohols where the hydroxyl group is attached to a primary carbon atom. Thus, it has the general structure, RCH2OH, where R is a hydrogen atom or an alkyl group.
electron affinity → elektronski afinitet
Electron affinity (EA) is the energy change occurring when an atom or molecule gains an electron to form a negative ion. For an atom or molecule X, it is the energy released for the electron-attachment reaction
This is often measured in electronvolts. Alternatively, the molar enthalpy change, ΔH, can be used.
enzyme → enzim
Enzyme is a protein that acts as a catalyst in biochemical reactions. Each enzyme is specific to a particular reaction or a group of similar reactions. Many require the association of certain nonprotein cofactors in order to function. The molecule undergoing a reaction (the substrate) binds to a specific active site on the enzyme molecule to form a short-lived intermediate: this greatly increases (by a factor of up to 1020) the rate at which the reaction proceeds to form the product.
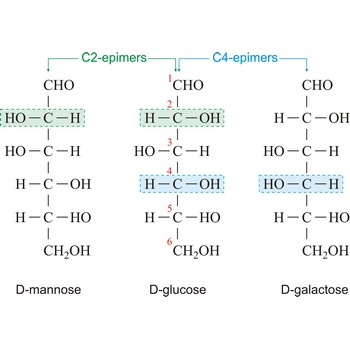
epimer → epimer
Epimers are diastereoisomers that have the opposite configuration at only one of two or more chiral centers present in the respective molecular entities. For example D-glucose and D-mannose, which differ only in the stereochemistry at C-2, are epimers, as are D-glucose and D-galactose (which differ at C-4).
primary amine → primarni amin
Primary amine is an amine on which there is only one alkyl group bonded, it is a weak base, and has a fish odour.
refractory metal → vatrostalni metal
Refractory metal is a metal having an extremely high melting point, for example tungsten, molybdenum, niobium, tantalum, and rhenium
saponification → saponifikacija
Saponification is a proces of hydrolysis of esters using hot sodium hydroxide solution to produce the salt of a carboxylic acid. Saponification usually refers to the hydrolysis of esters of fatty acids to manufacture soaps.
Citing this page:
Generalic, Eni. "Karboksilna skupina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table