salt fog test → ispitivanje u slanoj komori
Salt fog test is an accelerated corrosion test in which specimens are exposed to a fine mist of a solution usually containing sodium chloride (typically 5 %). Other contaminants can be added according to desired conditions. It is mainly used to determine the effectiveness of material finishes and protective coatings on materials. Salt-fog testing is also used to determine the effects of salt deposits on the electrical functions of electronic assemblies.
organoleptic testing → organoleptičko ispitivanje
Organoleptic testing of matter is a testing of matter and material using the senses of sight, smell, taste and touch, used usually when buying groceries.
salt fog chambers → slana komora
Salt fog chambers are designed for corrosive atmosphere testing. The samples being tested are inserted into the chamber and then the salt-containing solution is sprayed as a very fine fog mist over the samples. The temperature within the chamber is maintained constant (usually 35 °C). These test chambers are constructed of non-corrosive materials.
Wilson’s chamber → Wilsonova komora
Wilson’s chamber is used for detection of radioactive radiation. Wilson’s chamber has a glass cylinder filled with air that has been saturated with water vapour. Radioactive radiation in its way ionises molecules of gas which then function as centres on which water vapour condenses into very small drops, thereupon showing Tyndall’s effect, i.e. is they are visible as a bright trail.
seawater → more
Seawater is a complex mixture of 96.5 % water, 3.5 % salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates, and a few atmospheric gases. The world's oceans cover nearly 71 % (361 840 000 km2) of the Earth's surface (510 100 000 km2), with an average depth of 3 682.2 m.
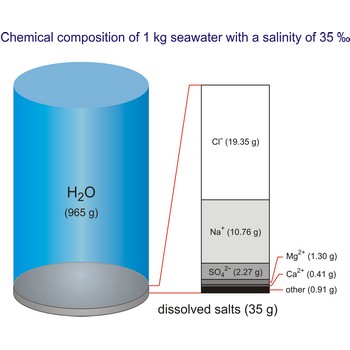
The density of seawater is higher than that of fresh water because of its higher salinity. Seawater's freezing point is lower than that of pure water and its boiling point is higher. The average salinity of the ocean is 35 ‰, which means that for every kilograms of water, there are 35 g of salt. The relative abundance of the major salts in seawater are constant regardless of the ocean. Only six elements and compounds comprise about 99 % of sea salts: chlorine (Cl-), sodium (Na+), sulfur (SO42-), magnesium (Mg2+), calcium (Ca2+), and potassium (K+).
atom marking → markiranje atoma
Atom marking is a process of infusing radioactive isotopes in live organism, with the purpose of revealing a way, diffusion, or a role of certain substance.
chlorinity → klorinitet
Originally chlorinity (symbol Cl) was defined as the weight of chlorine in grams per kilogram of seawater after the bromides and iodides had been replaced by chlorides. To make the definition independent of atomic weights, chlorinity is now defined as 0.3285233 times the weight of silver equivalent to all the halides.
The Mohr-Knudsen titration method served oceanographers for more than 60 years to determine salinity from chlorinity. This modification of the Mohr method uses special volumetric glassware calibrated directly in chlorinity units. The Mohr method uses potassium chromate (K2CrO4) as an indicator in the titration of chloride ions chloride (plus a small amount of bromide and iodide) with a silver nitrate (AgNO3) standard solution.
The other halides present are similarly precipitated.
A problem in the Mohr titration was that silver nitrate is not well suited for a primary standard. The Danish physicist Martin Knudsen (1871-1949) suggested that a standard seawater (Eau de mer Normale or Copenhagen Normal Water) be created and distributed to oceanographic laboratories throughout the world. This water was then used to standardize the silver nitrate solutions. In this way all chlorinity determinations were referred to one and the same standard which gave great internal consistency.
The relationship between chlorinity Cl and salinity S as set forth in Knudsen's tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
isotropic crystal → izotropni kristal
The measured properties of an isotropic crystal are independent of the axis of testing. Opposite of anisotropic.
radiography → radiografija
Radiography is nondestructive method of internal examination in which metal objects are exposed to a beam of X-ray or gamma radiation. Differences in thickness, density, or absorption caused by internal defects or inclusions are apparent in the shadow image produced on a fluorescent screen or photographic film placed behind the object.
Citing this page:
Generalic, Eni. "Ispitivanje u slanoj komori." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table