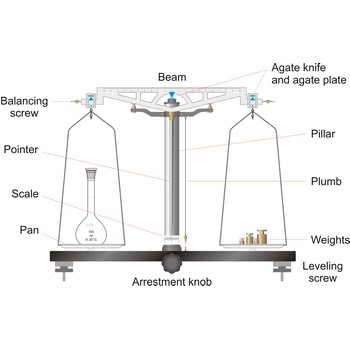
balance → vaga
Balance is an instrument to measure the mass (or weight) of a body. Balance beam type scales are the oldest type and measure weight using a fulcrum or pivot and a lever with the unknown weight placed on one end of the lever, and a counterweight applied to the other end. When the lever is balanced, the unknown weight and the counterweight are equal. The equal-arm balance consists of two identical pans hung from either end of a centrally suspended beam. The unequal-arm balance is made with one arm of the balance much longer than the other.
More modern substitution balances use the substitution principle. In this calibrated weights are removed from the single lever arm to bring the single pan suspended from it into equilibrium with a fixed counter weight. The substitution balance is more accurate than the two-pan device and enables weighing to be carried out more rapidly.
Electromagnetic force restoration balances also use a lever system but a magnetic field is used to generate the force on the opposite end of the lever and balance out the unknown mass. The current used to drive the magnetic coil is proportional to the mass of the object placed on the platform.
body-centered cubic lattice → prostorno centrirana kubična rešetka
Body-centered cubic lattice (bcc or cubic-I), like all lattices, has lattice points at the eight corners of the unit cell plus an additional points at the center of the cell. It has unit cell vectors a = b = c and interaxial angles α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the bcc structures the spheres fill 68 % of the volume. The number of atoms in a unit cell is two (8 × 1/8 + 1 = 2). There are 23 metals that have the bcc lattice.
enthalpy growth → prirast entalpije
Enthalpy growth (ΔH) is that part of energy of system which can be translated into heat (Q) with a permanent pressure.
environment → okolina
Environment (in thermodynamics) is the whole universe, except the system that is being observed.
fraction → udio
Fraction is a ratio of two quantities of the same kind, the numerator quantity applying to one constituent (or part) of the system and the denominator to the sum of quantities for all constituents (parts) of the system. When applied to mixtures fractions represent a group of three quantities: mass fraction, volume fraction and amount fraction (or mole fraction equal to the number fraction).
free energy → slobodna energija
Free energy is an energy that is actually available to do useful work. A decrease in free energy accompanies any spontaneous process. Free energy does not change for systems that are at equilibrium.
Born-Haber cycle → Born-Haberov kružni proces
Born-Haber cycle is a cycle of reactions used for calculating the lattice energies of ionic crystalline solids. For a compound MX, the lattice energy is the enthalpy of the reaction
The standard enthalpy of formation of the ionic solid is the enthalpy of the reaction
The cycle involves equating this enthalpy (which can be measured) to the sum of the enthalpies of a number of steps proceeding from the elements to the ionic solid. The steps are:
1) Atomization of the metal
2) Atomization of the nonmetal
3) Ionisation of the metal
This is obtained from the ionisation potential.
4) Ionisation of the nonmetal
This is electron affinity.
5) Formation of the ionic solids
Equation of the enthalpies gives
from which ΔHL can be found.
calomel electrode → kalomel elektroda
Calomel electrode is a type of half cell in which the electrode is mercury coated with calomel (Hg2Cl2) and the electrolyte is a solution of potassium chloride and saturated calomel. In the calomel half cell the overall reaction is
Table: Dependence of potential of calomel electrode upon temperature and concentration of KCl according to standard hydrogen electrode
| Potential vs. SHE / V | |||
|---|---|---|---|
| t / °C | 0.1 mol dm-3 | 3.5 mol dm-3 | sat. solution |
| 15 | 0.3362 | 0.254 | 0.2511 |
| 20 | 0.3359 | 0.252 | 0.2479 |
| 25 | 0.3356 | 0.250 | 0.2444 |
| 30 | 0.3351 | 0.248 | 0.2411 |
| 35 | 0.3344 | 0.246 | 0.2376 |
Citing this page:
Generalic, Eni. "Inercijski referentni sustavi." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table