salinity → salinitet
Salinity (S) is a measure of the quantity of dissolved salts in seawater. It is formally defined as the total amount of dissolved solids in seawater in parts per thousand (‰) by weight when all the carbonate has been converted to oxide, the bromide and iodide to chloride, and all organic matter is completely oxidized.
Chlorinity is the oldest of the salinity measures considered and is still a corner-stone in the study of dissolved material in seawater. Based on the principle of constant relative proportions it provides a measure of the total amount of dissolved material in seawater in terms of the concentration of halides. The relationship between chlorinity (Cl) and salinity as set forth in Knudsen’s tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
Practical Salinity (SP) was introduced as a replacement for Chlorinity. Practical Salinity is is relatively easy to measure using standard conductometers, measurements are more precise and less time consuming than measurements of Chlorinity and accurate measurements can even be made in situ. Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution).
Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". For most purposes one can assume that the psu and the ‰, are synonymous.
The global average salinity of ocean waters is about 35 ‰, that is, about 35 g of solid substances are dissolved in 1 kg of seawater.
Schrodinger equation → Schrodingerova jednadžba
Schrödinger equation is the basic equation of wave mechanics which, for systems not dependent on time, takes the form:
where Ψ is the wavefunction, V is the potential energy expressed as a function of the spatial coordinates, E its total energy, ![]() 2 is the Laplacian operator, h is Planck’s constant, and m is the mass.
2 is the Laplacian operator, h is Planck’s constant, and m is the mass.
second-order reactions → reakcije drugog reda
Second-order reaction is a reaction with a rate law that is proportional to either the concentration of a reactant squared, or the product of concentrations of two reactants.
For a general unimolecular reaction,
The reaction rate expression for a second order reaction is
If assumed that the concentration of reactant A is [A]o at t=0 and [A] at time T, the variables in the rate equation and integrate can be separated. The integrated rate law for a second-order reaction can be easily shown to be
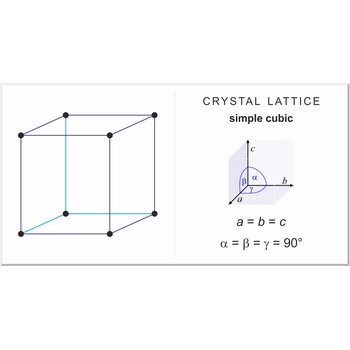
simple cubic lattice → jednostavna kubična rešetka
Simple or primitive cubic lattice (sc or cubic-P) has one lattice point at the each corner of the unit cell. It has unit cell vectors a = b = c and interaxial angels α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the sc structures the spheres fill 52 % of the volume. The number of atoms in a unit cell is one (8×1/8 = 1). This is only one metal (α-polonium) that have the sc lattice.
sol → sol
Sols are dispersions of small solid particles in a liquid. The particles may be macromolecules or may be clusters of small molecules. Lyophobic sols are those in which there is no affinity between the dispersed phase and the liquid (e.g. silver chloride dispersed in water). Lyophobic sols are inherently unstable, in time the particles aggregate, and form a precipitate. Lyiophilic sols, on the other hand, are more like true solutions in which the solute molecules are large and have an affinity for the solvent (e.g. starch in water). Association colloids are systems in which the dispersed phase consists of clusters of molecules that have lyophobic and lyophilic parts (e.g. soap in water).
stoichiometry → stehiometrija
Stoichiometry is the relative proportions elements from compounds or in which substances react. Every chemical reaction has its characteristic proportions. For example, when methane unites with oxygen in complete combustion, 1 mol of methane requires 2 mol of oxygen.
At the same time, 1 mol of carbon dioxide and 2 mol of water are formed as reaction products.
Alternatively, 16 g of methane and 64 g of oxygen produce 44 g of carbon dioxide and 36 g of water.
The stoichiometric relationship between the products and reactants can be used to in calculations.
sugar → šećer
Sugar is any of a group of water-soluble carbohydrates of relatively low molecular weight and typically having a sweet taste. The group comprises mainly monosaccharides (glucose, fructose, galactose), disaccharides (sucrose, lactose, maltose), and trisaccharides (raffinose). Many monosaccharides and disaccharides fairly commonly found in nature bear names reflecting the source from which they were first isolated. For example, glucose is also known as grape sugar, lactose as milk sugar, and maltose as malt sugar. In everyday usage, the name is often used to refer specifically to sucrose (table sugar, cane sugar, beet sugar).
sulfur → sumpor
Sulfur has been known since ancient times. The origin of the name comes from the Sanskrit word sulvere meaning sulphur; also from the Latin word sulphurium meaning sulphur. It is pale yellow, odourless, brittle solid, which is insoluble in water but soluble in carbon disulfide. Sulfur is found in pure form and in ores like cinnabar, galena, sphalerite and stibnite. Pure form is obtained from underground deposits by the Frasch process. Used in matches, gunpowder, medicines, rubber and pesticides, dyes and insecticides. Also for making sulfuric acid (H2SO4).
superoxide → superoksid
Superoxides are binary compounds containing oxygen in the -½ oxidation state. Sodium superoxide (NaO2) can be prepared with high oxygen pressures, whereas the superoxides of rubidium, potassium, and cesium can be prepared directly by combustion in air. These compounds are yellow to orange paramagnetic solids. Superoxide ion, O2-, has an unpaired electron, is not particularly stable, and spontaneously decomposes into peroxide over time.
They are strong oxidising agents that vigorously hydrolyze (react with water) to produce superoxide and oxygen gas.
Citing this page:
Generalic, Eni. "Having such a good time with me." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table