retention time → retencijsko vrijeme
Retention time is time between incentive (sample injection) and recall (appearance of signal on detector).
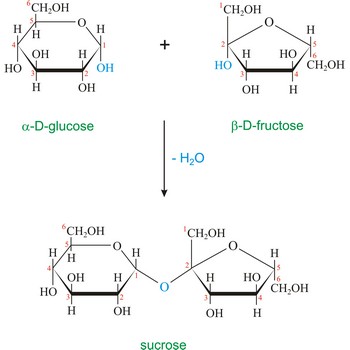
sucrose → saharoza
Sucrose (saccharose), or ordinary table sugar, is a disaccharide in which α-D-glucopyranose and β-D-fructofuranose are joined at their anomeric carbons by a glycosidic bond. There are no hemiacetals remaining in the sucrose and therefore sucrose is not a reducing sugar and does not exhibit mutarotation. Sugar is a white crystalline sweet compound found in many plants and extracted from sugar cane and sugar beet. It is used as a sweetening agent in food and drinks. If heated to 200 °C, sucrose becomes caramel. When sucrose is hydrolyzed it forms an equimolar mixture of glucose and fructose. This mixture of monosaccharides is called invert sugar. Honeybees have enzymes called invertases that catalyze the hydrolysis of sucrose. Honey, in fact, is primarily a mixture of glucose, fructose, and sucrose.
extraction → ekstrakcija
Extraction is the separation of a component from its mixture by selective solubility. When a solution of one substance in one solvent is brought in with another solvent dissolved substance will distribute between the two solutants because of different solubility. Extraction is an efficient and fast method used for separating and concentrating matters. Extraction is best done several times in a succession, with smaller amount of solvent in it the matter is better dissolved. For example, caffeine can be separated from coffee beans by washing the beans with supercritical fluid carbon dioxide; the caffeine dissolves in the carbon dioxide, but flavour compounds do not. Vanillin can be extracted from vanilla beans by shaking the beans with an organic solvent, like ethanol.
gasoline → motorni benzin
Gasoline is a complex mixture of volatile hydrocarbons that may have between 5 to 12 carbons. The major components are branched-chain paraffins, cycloparaffins, and aromatics. Gasoline is most often produced by the fractional distillation of crude oil as the fraction of hydrocarbons in petroleum boiling between 30 °C and 200 °C. The quality of a fuel is measured with its octane number. Octane number is the measure of the resistance of gasoline against detonation or preignition of the fuel in the engine. The higher the octane number, the more compression the fuel can withstand before detonating. The octane number is determined by comparing the characteristics of a gasoline to isooctane with good knocking properties (octane number of 100) and heptane with bad (octane number of 0).
gold → zlato
Gold has been known since ancient times. The origin of the name comes from the Latin word aurum meaning gold. It is soft, malleable, bright yellow metal. Unaffected by air, water, alkalis and most acids. Gold is found in veins in the crust, with copper ore and native. Used in electronics, jewellery and coins. It is a good reflector of infrared radiation, so a thin film of gold is applied to the glass of skyscrapers to reduce internal heating from sunlight.
silver → srebro
Silver has been known since ancient times. The origin of the name comes from the Latin word argentum meaning silver. It is silvery-ductile and malleable metal. Stable in water and oxygen. Reacts with sulfur compounds to form black sulfides. Silver is found in ores called argentite (AgS), light ruby silver (Ag3AsS3), dark ruby silver (Ag3SbS3) and brittle silver. Used in alloys for jewellery and in other compounds for photography. It is also a good conductor, but expensive.
accelerated corrosion test → ubrzana korozija
Accelerated corrosion test is method designed to approximate, in a short time, the deteriorating effect under normal long-term service conditions.
acceleration → akceleracija
If a point-like object undergoes a change in velocity Δv=vf-vi in time Δt=tf-ti (indexes i and f stand for initial and final instant as well as for initial and final velocity) its average acceleration, a is defined as
The instantaneous acceleration, a, is obtained from the average acceleration by shrinking the time interval Δt towards zero. The average acceleration approaches a limiting value, which is the acceleration of a given instant:
Acceleration is a vector quantity. SI unit for acceleration is m s-2.
Acheson process → Achesonov proces
Acheson process is an industrial process to synthesize graphite and silicon carbide (carborundum), named after its inventor the American chemist Edward Goodrich Acheson (1856-1931). In this process, a solid-state reaction between pure silica sand (SiO2) and petroleum coke (C) at very high temperature (more than 2500 °C) leads to the formation of silicon carbide under the general reaction:
While studying the effects of high temperature on carborundum, Acheson had found that silicon vaporizes at about 4150 °C, leaving behind graphitic carbon.
adrenaline → adrenalin
Adrenaline was the first naturally produced hormone to be isolated in its pure state. It is known as epinephrine, but its chemical name is 1-[3,4-dihydroxyphenol]-2-methylaminoethanol. Adrenaline is a hormone, produced by the medulla of the adrenal glands, that increases heart activity, improves the power and prolongs the action of muscles, and increases the rate and depth of breathing to prepare the body for "fright, flight, or fight". At the same time it inhibits digestion and excretion.
Citing this page:
Generalic, Eni. "Having such a good time with me." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table