metre → metar
Metre (m) is the SI base unit of length.
The meter is the length of the path travelled by light in vacuum during a time interval of 1/299 792 458 s.
This definition, adopted by the General Conference on Weights and Measure in October 1983, replaced the 1967 definition based on the krypton lamp.
microscope → mikroskop
Microscope is an instrument that produces enlarged images of small objects. The optical microscopes (light microscope) use visible light and a system of lenses to magnify images. Typical magnification of a light microscope is up to 1500× ("1500 times")with a theoretical resolution limit of around 200 nm. Instead of using light, electron microscopes transmit a beam of electrons through, or onto the surface of, a specimen. An electron beam has a much shorter wavelength than does light, and can reveal structures as small as 2 nm.
non-metal → nemetal
Non-metals are defined as elements that are not metals.
Their physical properties generally include:
- They are poor conductors.
- They are brittle, not ductile in their solid state.
- They show no metallic lustre.
- They may be transparent or translucent.
- They have low density.
- They form molecules which consists of atoms covalently bonded; the noble gases are monoatomic.
Their chemical properties are generally:
- They usually have four to eight valence electrons.
- They have high electron affinities (except the noble gases)
- They are good oxidising agents (except the noble gases)
- They have hydroxides which are acidic (except the noble gases)
- They are electronegative.
Ostwald’s viscometer → Ostwaldov viskozimetar
Ostwald viscometer, also known as U-tube viscometer or capillary viscometer is a device used to measure the viscosity of the liquid with a known density. The method of determining viscosity with this instrument consists of measuring the time for a known volume of the liquid (the volume contained between the marks A and B) to flow through the capillary under the influence of gravity. Ostwald viscometers named after the German chemist Wilhelm Ostwald (1853-1932).
The instrument must first be calibrated with materials of known viscosity such as pure (deionized) water. Knowing the value of viscosity of one liquid, one can calculate the viscosity of other liquid.
where η1 and η2 are viscosity coefficients of the liquid and water, and ρ1 and ρ2 are the densities of liquid and water, respectively.
palladium → paladij
Palladium was discovered by William Hyde Wollaston (England) in 1803. Named after the asteroid Pallas which was discovered at about the same time and from the Greek name Pallas, goddess of wisdom. It is soft, malleable, ductile, silvery-white metal. Resists corrosion; dissolves in oxidizing acids. Absorbs hydrogen. Metal dust is combustible. Palladium is obtained with platinum, nickel, copper and mercury ores. Used as a substitute for silver in dental items and jewellery. The pure metal is used as the delicate mainsprings in analog wristwatches. Also used in surgical instruments and as catalyst.
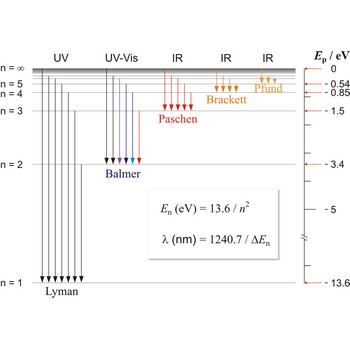
Paschen series → Paschenova serija
Paschen series are the series of lines in the spectrum of the hydrogen atom which corresponds to transitions between the state with principal quantum number n = 3 and successive higher states.
radioactive series → radioaktivni niz
Radioactive series is a sequence of nuclides formed by successive radioactive decays until a stable decay product, the end product, is formed. A famous example of a radioactive series is the decay of uranium, which through a series of steps decays into stable lead.
pipette → pipeta
Pipettes are glass tubes which are tapers towards at both ends into narrow opened tubes. According to their design two types of pipettes can be distinguished:
Volumetric pipettes
Volumetric pipettes (transfer or belly pipette) are used in volumetric analysis, when there is a need for taking exact smaller volume of a sample solution or reagent. The upper tube of volumetric pipette has a ringlike marking (mark) which marks its calibrated volume. Pipettes calibrated to deliver (TD or Ex) the indicated volume. By sucking in (with mouth, propipette or a water pump) the liquid is pulled in a little bit above the mark and the opening of the pipet is closed with a forefingertip. Outer wall of pipet is wiped and, with a slight forefinger loosening, the liquid is released until it reaches the mark. Mark must figure as a tangent on a lower edge of the liquid meniscus. A pipette is emptied out by lifting the forefinger off and letting the liquid flow out of the pipette freely. After another 15 s and the tip of the pipette is pulled onto the inner wall of the vessel. It is absolutely forbidden to blow out the contents of the pipette
Graduated pipettes
Graduated pipettes (Mohr pipette) have a scale divided into units of one and of 1/10th of a millilitre. Because of their wide necks it is less accurate than the volumetric pipette. They are used when taking volume of solutions in which accuracy does not have to be very high. They are filled in the same way as volumetric ones and liquid can be gradually released.
resistivity → električna otpornost
Electrical resistivity, or specific resistance (ρ) is the electric field strength divided by the current density when there is no electromotive force in the conductor. Resistivity is an intrinsic property of a material. Materials with low resistivity are good conductors of electricity and materials with high resistivity are good insulators.
For a conductor of uniform cross section with area A and length L, and whose resistance is R, the resistivity is given by
The SI unit is Ω m.
Citing this page:
Generalic, Eni. "Having such a good time with me." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table