supercritical fluid extraction → superkritična fluidna ekstrakcija
Supercritical fluid extractions (SFE) have solvating powers similar to liquid organic solvents, but with higher diffusivities, lower viscosity, and lower surface tension. The main advantages of using supercritical fluids for extractions is that they are inexpensive, contaminant free, and less costly to dispose safely than organic solvents. For non-destructive isolation choose SFE, which is simply the best technology for sensitive raw materials. For these reasons supercritical carbon dioxide (scCO2) is the reagent used to extract caffeine from coffee and tea. Its gaslike behavior allows it to penetrate deep into the green coffee beans, and it dissolves from 97 % to 99 % of the caffeine present.
volumetric pipette → prijenosna pipeta
Volumetric pipettes (transfer or belly pipette) are used in volumetric analysis, when there is a need for taking exact smaller volume of a sample solution or reagent. The upper tube of volumetric pipette has a ringlike marking (mark) which marks its calibrated volume. Pipettes calibrated to deliver (TD or Ex) the indicated volume. By sucking in (with mouth, propipette or a water pump) the liquid is pulled in a little bit above the mark and the opening of the pipet is closed with a forefingertip. Outer wall of pipet is wiped and, with a slight forefinger loosening, the liquid is released until it reaches the mark. Mark must figure as a tangent on a lower edge of the liquid meniscus. A pipette is emptied out by lifting the forefinger off and letting the liquid flow out of the pipette freely. After another 15 s and the tip of the pipette is pulled onto the inner wall of the vessel. It is absolutely forbidden to blow out the contents of the pipette.
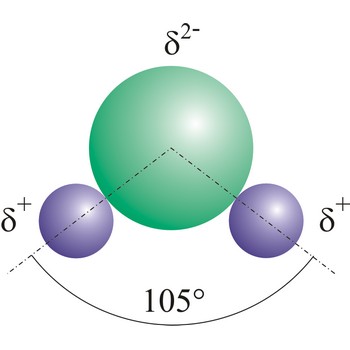
water → voda
Water (H2O) (dihydrogen oxide) is a binary compound that occurs at room temperature as a clear colorless odorless tasteless liquid; freezes into ice below 0 °C and boils above 100 °C. Water is a chemical compound which is essential for living organisms and it is widely used as a solvent.
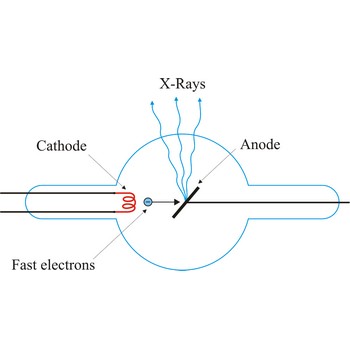
X-ray tube → rendgenska cijev
X-ray tube is a cathode ray tube that focuses energetic streams of electrons on a metal target, causing the metal to emit X-rays. The basic principle of the X-ray tube has not changed significantly since Roentgen's 1895 discovery. Current applied to a metal cathode (about 50 000 V) produces free electrons. The X-rays are produced when the rapidly moving electrons are suddenly stopped as they strike the metal target of the tube.
X-rays → X-zrake
X-rays are electromagnetic radiation of shorter wavelength than ultraviolet radiation (10-11 m to 10-9 m or 0.01 nm to 1 nm) produced by bombardment of atoms by high-quantum-energy particles. X-rays can pass through many forms of matter and they are therefore used medically and industrially to examine the internal structure.

Chitosan → Kitozan
Chitosan is a linear polysaccharide composed of randomly distributed N-acetyl D-glucosamine and D-glucosamine units. It can be easily derived from partial deacetylation of natural polymer chitin. At a minimum deacetylization level of 60 % (amount of free amino groups in the polymer) it is considered to be chitosan. Thanks to the amino groups of D-glucosamine, chitosan can be protonated and turned into polycation, which is one of the sources of unique properties of chitosan as biopolymer, like aqueous solubility, antibacterial properties, biodegradability with non-toxic residues and biocompatibility.
Citing this page:
Generalic, Eni. "Google translate freee." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table