alkenes → alkeni
Alkenes are acyclic branched or unbranched hydrocarbons having one or more double carbon-carbon bonds in their molecules. In the systematic chemical nomenclature, alkene names end in the suffix -ene. The general formula is CnH(2n+2)-2x were x is the number of double bonds. Alkenes that have only one double bond form a homologous series: ethene (ethylene), CH2=CH2, propene, CH3CH2=CH2, etc. Alkenes typically undergo addition reactions to the double bond.
alkynes → alkini
Alkynes (acetylenes) are acyclic branched or unbranched hydrocarbons having one or more triple carbon-carbon bond. In the systematic chemical nomenclature alkyne names end in the suffix -yne. The general formula is CnH(2n+2)-4x were x is the number of triple bonds. Alkynes that have only one triple bond form a homologous series: ethyne (acetylene), CH≡CH, propyne, CH3CH≡CH, etc. Like alkenes, alkynes undergo addition reaction.
butane → butan
Butane is a gaseous hydrocarbon C4H10 obtained from petroleum (refinery gas or by cracking higher hydrocarbons). The fourth member of the alkane series, it has a straight chain of carbon atoms and is isomeric with 2-methylpropane, formerly called isobutene. It can easily be liquefied under pressure and is supplied into cylinders for use as a fuel gas. It is also a raw material for making buta-1, 3-diene for synthetic rubber.
diffraction grating → difrakcijska rešetka
Diffraction grating is a series of slits used to separate an incident wave into its component wavelengths by directionally separating their diffraction maxima.
Allihn condenser → Allihnovo hladilo
Allihn condenser or bulb condenser consists of an outer water jacket and the inner glass tube with a series of spherical bubbles to maximize the thermal contact with the cooling water. It is named after its inventor, the German chemist Felix Richard Allihn (1854-1915).
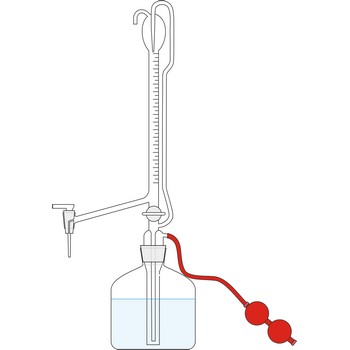
automatic burette → automatska bireta
Automatic burette is used for series of tests. It is connected with a bottle which contains the titration solution. The air is pumped into the bottle by a small rubber air pump, created the pressure in the bottle which the rises the solution to the top of burette. When the the burette is full, the valve is released, the pressure in the bottle falls and the burette automatically sets itself to zero. Work with automatic burettes is by far faster and the consumption of standard solution is smaller.
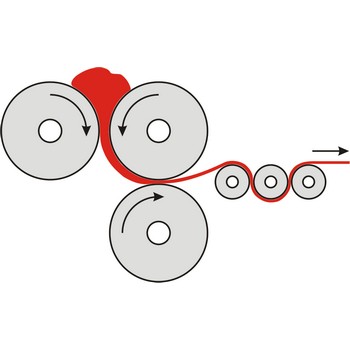
calendering → kalandriranje
Calendering is the process of forming materials to make a film/sheet by passing them through a series of hot rollers.
electrode of the third kind → elektroda trećeg reda
Electrode of the third kind is a metal electrode assembly with the equilibrium potential being a function of the concentration of a cation, other than the cation of the electrode metal, in the solution. The assembly consists of a metal in contact with two slightly soluble salts (one containing the cation of the solid metal, the other the cation to be determined, with both salts having a common anion) immersed in a solution containing a salt of the second metal (e.g., zinc metal--zinc oxalate--calcium oxalate--calcium salt solution). The potential of the metal is controlled by the concentration of its cation in the solution, but this is controlled by the anion concentration in the solution through the solubility product of the slightly soluble metal salt, which, in turn is controlled by the concentration of the cation of the second slightly soluble salt. These electrodes are very sluggish and unstable due to a series of equilibria to be established to produce a stable potential.
fatty acid → masna kiselina
Fatty acids are aliphatic monocarboxylic acids characterized by a terminal carboxyl group (R-COOH). The higher members of this series of acids occur in nature in the combined form of esters of glycerol (fats), and hence all acids of this family are called fatty acids. Natural fatty acids commonly have a chain of 4 to 28 carbons (usually unbranched and even-numbered), which may be saturated or unsaturated. The most important of saturated fatty acids are butyric (C4), lauric (C12), palmitic (C16), and stearic (C18). The most common unsaturated acids are oleic, linoleic, and linolenic (all C18).
The physical properties of fatty acids are determined by the chain length, degree of unsaturation, and chain branching. Short-chain acids are pungent liquids, soluble in water. As the chain length increases, melting points are raised and water-solubility decreases. Unsaturation and chain branching tend to lower melting points.
Citing this page:
Generalic, Eni. "Fibonacci series logic." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table