Lewis acid → Lewisova kiselina
Lewis acid is an agent capable of accepting a pair of electrons to form a coordinate bond.
Lewis base → Lewisova baza
Lewis base is an agent capable of donating a pair of electrons to form a coordinate bond.
Lewis, Gilbert N. → Lewis, Gilbert N.
Gilbert Newton Lewis (1875-1946) is an American chemist whose theory of the electron pair fostered understanding of the covalent bond and extended the concept of acids and bases.
ligand field theory → teorija ligandnog polja
Ligand field theory is a description of the structure of crystals containing a transition metal ion surrounded by nonmetallic ions (ligands). It is based on the construction of molecular orbitals involving the d-orbitals of the central metal ion and combinations of atomic orbitals of the ligands.
dipole → dipol
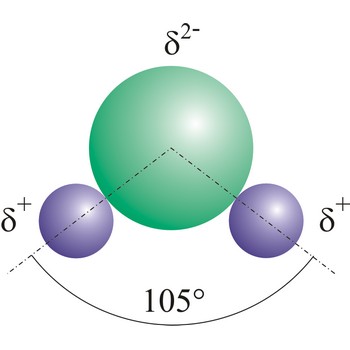
Dipole is a pair of separated opposite electric charges. Electric dipole is an assemblage of atoms or subatomic particles having equal electric charges of opposite sign separated by a finite distance. In the case of HCl, the electrons are attracted towards the more electronegative chlorine atom.
dipole molecule → dipolna molekula
Dipole molecules are created when mutual electronic pair at covalent bond is asymmetrical. If different atoms are bonded by a covalent bond, which can have different electron affinity, then the the atom with greater electron affinity will attract the electron pairs more strongly. In this way an asymmetrical distribution of negative charge appears in a molecule, so one part of the molecule becomes relatively negatively (the one closer to the electron pair) and the other becomes relatively positively charged.
electrochemical cell → elektrokemijski članak
Electrochemical cell is a device that converts chemical energy into electrical energy or vice versa when a chemical reaction is occurring in the cell. It consist of two electronically conducting phases (e.g., solid or liquid metals, semiconductors, etc) connected by an ionically conducting phase (e.g. aqueous or non-aqueous solution, molten salt, ionically conducting solid). As an electric current passes, it must change from electronic current to ionic current and back to electronic current. These changes of conduction mode are always accompanied by oxidation/reduction reactions.
An essential feature of the electrochemical cell is that the simultaneously occurring oxidation-reduction reactions are spatially separated. E.g., in a spontaneous chemical reaction during the oxidation of hydrogen by oxygen to water, electrons are passed directly from the hydrogen to the oxygen.
In contrast, in the spontaneous electrochemical reaction in a galvanic cell the hydrogen is oxidised at the anode by transferring electrons to the anode and the oxygen is reduced at the cathode by accepting electrons from the cathode. The ions produced in the electrode reactions, in this case positive hydrogen ions and the negative hydroxyl (OH-) ions, will recombine in the solution to form the final product of the reaction: water. During this process the electrons are conducted from the anode to the cathode through an outside electric circuit where the electric current can drive a motor, light a light bulb, etc. The reaction can also be reversed: water can be decomposed into hydrogen and oxygen by the application of electrical power in an electrolytic cell.
London’s force → Londonova sila
London’s force is an intermolecular attractive force that arises from a cooperative oscillation of electron clouds on a collection of molecules at close range.
metalloid → polumetal
Metalloid (semimetal) is any of a class of chemical elements intermediate in properties between metals and nonmetals. The classification is not clear cut, but typical metalloids are boron (B), silicon (Si), germanium (Ge), arsenic (As), and tellurium (Te). They are electrical semiconductors and their oxides are amphoteric.
nucleophile → nukleofil
Nucleophiles are negatively charged or bear a partial negative charge. Examples are lone pairs or a hydroxide ion.
Citing this page:
Generalic, Eni. "Elektronska konfiguracija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table