indicator → indikator
Indicator is a substance used to show the presence of a chemical substance or ion by its colour. Acid-base indicators are compounds, such as phenolphtaleine and methyl orange, which change colour reversibly, depending on whether the solution is acidic or basic. Oxidation-reduction indicators are substances that show a reversible colour change between oxidised and reduced forms.
iron → željezo
Iron has been known since ancient times. The origin of the name comes from the Latin word ferrum meaning iron. It is malleable, ductile, silvery-white metal. Exposed surfaces form red-brown oxides. Forms very strong alloys (steel). Ferromagnetic. Metal dust flammable. Fourth most abundant element in the earth’s crust. Iron is obtained from iron ores. Pure metal produced in blast furnaces by layering limestone, coke and iron ore and forcing hot gasses into the bottom. This heats the coke red hot and the iron is reduced from its oxides and liquefied where it flows to the bottom. Iron is the most common metal in human society. More than 90 % of all metal refined in the world is iron. Used in steel and other alloys. It is the chief constituent of hemoglobin which carries oxygen in blood vessels. Its oxides are used in magnetic tapes and disks.
lactose → laktoza
Lactose (milk sugar) is a disaccharide comprising one glucose molecule linked to a galactose molecule by an β(1→4)-glycosidic linkage. Lactose has a beta acetal. Lactose is manufactured by the mammary gland and occurs only in milk (from 4 % to 7 %). Lactose intolerance is a common medical condition that results in diarrhea, abdominal pain, and flatulence and is caused by reduced or absent activity of enzyme lactase.
Like cellobiose and maltose, lactose is a reducing sugar. All reducing sugar undergo mutarotation in aqueous solution. The equilibrium mixture at 20 °C is composed of 62.7 % β-lactose (β-D-galactopyranosyl-(1→4)-β-D-glucopyranose) and 37.3 % α-lactose (β-D-galactopyranosyl-(1→4)-α-D-glucopyranose).
Knudsen burette → Knudsenova bireta
Knudsen's automatic bulb-burette, developed by the Danish physicist Martin Knudsen (1871-1949), is designed in a way that even routine field analysis in a boat laboratory would provide highly accurate measurements. The burette is filled with a mixture of silver nitrate from reservoir R, located above the burette, by opening the A valve. When the solution crosses the three-way C valve the A valve is closed preventing further solution flow in to the burette. Any extra solution is caught in the W bowl. Turn the C valve, which marks the zero on the scale, in order to allow atmospheric air to enter the burette. Since most open-ocean samples lie in a relatively small chlorinity range, the burette is designed so that much of its capacity is in the bulb (B). This allows the titration to be quick (by quickly releasing contents from the B area) and reduces the error that occurs from the slow drainage along the inner wall of the burette.
Each millimeter is divided in to twenty parts (double millimeter division of the Knudsen burette) which allows for highly accurate measurements (the scale is read up to a precision of 0.005 mL). From 0 to 16 the burette isn't divided, that usually starts from 16 and goes until 20.5 or 21.5. A single double millimeter on a Knudsen burette scale corresponds to one permille of chloride in the seawater sample. This burette can be used for titration of water from all of the oceans and seas, with the exemptions being areas with very low salinity (e.g. the Baltic Sea) and river estuaries which require the use of normal burettes.
metal → metal
Metals are materials in which the highest occupied energy band (conduction band) is only partially filled with electrons.
Their physical properties generally include:
- They are good conductors of heat and electricity. The electrical conductivity of metals generally decreases with temperature.
- They are malleable and ductile in their solid state.
- They show metallic lustre.
- They are opaque.
- They have high density.
- They are solids (except mercury)
- They have a crystal structure in which each atom is surrounded by eight to twelve near neighbours
Their chemical properties generally are:
- They have one to four valence electrons.
- They have low ionisation potentials; they readily lose electrons.
- They are good reducing agents.
- They have hydroxides which are bases or amphoteric.
- They are electropositive.
Metallic characteristics of the elements decrease and non-metallic characteristics increase with the increase of valence electrons. Also metallic characteristics increase with the number of electron shells. Therefore, there is no sharp dividing line between the metals and non-metals.
Of the 114 elements now known, only 17 show primarily non-metallic characteristics, 7 others are metalloids, and 89 may be classed as metals.
monosaccharide → monosaharid
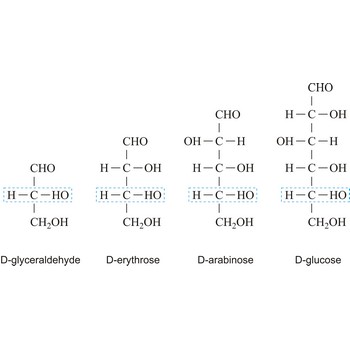
Monosaccharides are carbohydrates, with the general formula Cn(H2O)n, that cannot be decomposed to a simpler carbohydrates by hydrolysis.
Depending on whether the molecule contains an aldehyde group (-CHO) or a ketone group (-CO-) monosaccharide can be a polyhydroxy aldehyde (aldose) or a polyhydroxy ketone (ketose). These aldehyde and ketone groups confer reduction properties on monosaccharides. They are also classified according to the number of carbon atoms they contain: trioses have three carbon atoms, tetroses four, pentoses five, hexoses six, heptoses seven, etc. These two systems of classification are often combined. For example, a six-carbon polyhydroxy aldehyde such as D-glucose is an aldohexose, whereas a six-carbon polyhydroxy ketone such as D-fructose is a ketohexose.
The notations D and L are used to describe the configurations of carbohydrates. In Fischer projections of monosaccharides, the carbonyl group is always placed on top (in the case of aldoses) or as close to the top as possible (in the case of ketoses). If the OH group attached to the bottom-most asymmetric carbon (the carbon that is second from the bottom) is on the right, then the compound is a D-sugar. If the OH group is on the left, then the compound is an L-sugar. Almost all sugars found in nature are D-sugars.
Monosaccharides can exist as either straight-chain or ring-shaped molecules. During the conversion from straight-chain form to cyclic form, the carbon atom containing the carbonyl oxygen, called the anomeric carbon, becomes a chiral center with two possible configurations (anomers), α and β. When the stereochemistry of the first carbon matches the stereochemistry of the last stereogenic center the sugar is the α-anomer when they are opposite the sugar is the β-anomer.
Nernst’s electrode potential equation → Nernstova jednadžba za elektrodni potencijal
For general reaction of some redox system
dependence of electrode potential of redox system upon activity of oxidised and reduced form in solution is described in Nernst’s equation for electrode potential:
where E = to electrode potential of redox system
E° = standard electrode potential of redox system
R = universal gas constant
T = thermodymical temperature
F = Faraday’s constant
z = number of electrons exchanged in redox reaction
aO = activity of oxidised form
aR = activity of reduced form
n = stechiometrical coefficient of oxidised form
m = stechiometrical coefficient of reduced form
nuclear reactor → nuklearni reaktor
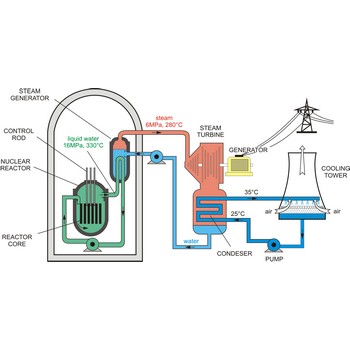
Nuclear reactor is an assembly of fissionable material (uranium-235 or plutonium-239) designed to produce a sustained and controllable chain reaction for the generation of electric power.
The essential components of a nuclear reactor are:
- The core, metal rods containing enough fissionable material to maintain a chain reaction at the necessary power level (as much as 50 t of uranium may be required).
- A source of neutrons to initiate the reaction (such as a mixture of polonium and beryllium)
- A moderator to reduce the energy of fast neutrons for more efficient fission (material such as graphite, beryllium, heavy water, and light water are used)
- A coolant to remove the fission-generated heat (water, sodium, helium, and nitrogen may be used)
- A control system such as rods of boron or cadmium that have high capture cross sections (to absorb neutrons)
- Adequate shielding, remote-control equipment, and appropriate instrumentation are essential for personnel safety and efficient operation.
poison → otrov
Poisons are substance, which upon contact or being introduced into an organism, impair or prevent normal metabolic processes from taking place, thus altering the normal functioning of organs or tissues.
Poisons are molecules or material that tends to collect on a catalyst surface, blocking access to active sites or destroying their activities.
Poisons are substance that can reduce a nuclear reaction by absorbing neutrons, thereby preventing more fission. If enough poisons are present in a reactor core, the chain reaction will die out.
polysaccharide → polisaharid
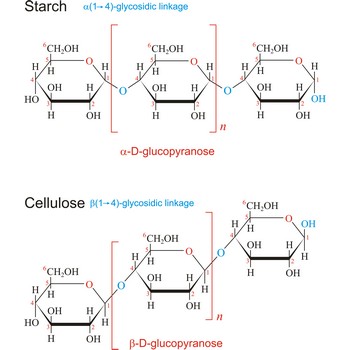
Polysaccharides are compounds consisting of a large number of simple sugars (monosaccharides) linked together by glycosidic bonds. When polysaccharides are composed of a single monosaccharide building block, they are termed homopolysaccharides. Heteropolysaccharides contain two or more different types of monosaccharide. Polysaccharides may have molecular weights of up to several million and are often highly branched. Since they have only the one free anomeric -OH group at the end of a very long chain, polysaccharides aren’t reducing sugars and don’t show noticeable mutarotation. The most common polysaccharides are cellulose, starch, and glycogen.
Citing this page:
Generalic, Eni. "Elektroda drugog reda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table