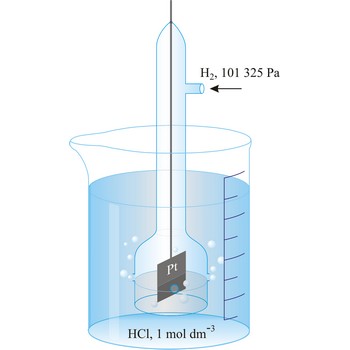
Jones’s reductor → Jonesov reduktor
Jones’s reductor is a column filled with zinc amalgam. It is used for analyte reduction.
redox potential → redoks potencijal
Redox potential is the potential of a reversible oxidation-reduction electrode measured with respect to a reference electrode, corrected to the hydrogen electrode, in a given electrolyte.
redox reaction → redoks-reakcija
Redox reaction is an oxidation-reduction reaction is a reaction in which one or more electrons are transferred. When an atom, ion, or molecule loses one or more electrons, it is oxidised. When an atom, ion, or molecule gains one or more electrons, it is reduced.
redox titration → redoks-titracija
Redox titration (oxidation-reduction titration) is a titration based on a redox reaction. For example, iron in water can be determined by converting dissolved iron to Fe2+ and titrating the solution with potassium permanganate (KMnO4), a powerful oxidising agent.
reducing agent → redukcijsko sredstvo
Reducing agent may be defined in various ways, depending upon the context in which the phrase is used. In broad terms it is often taken to mean a chemical which can act as an electron donor. Thus, in the reaction:
the zinc is being reduced (gaining electrons) by reaction with the iron cations; the Fe2+ in this instance is acting as a reducing agent.
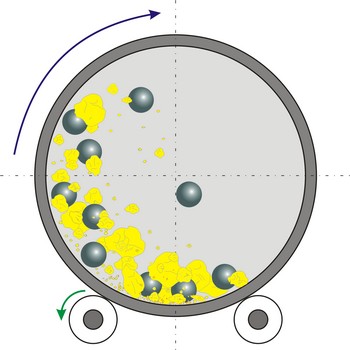
ball mill → kuglični mlin
Ball mill is a grinder for reducing hard materials to powder. The grinding is carried out by the pounding and rolling of a charge of steel or ceramic balls carried within the cylinder. The cylinder rotates at a relatively slow speed, allowing the balls to cascade through the mill base, thus grinding or dispersing the materials.
Type of ball mills, centrifugal and planetary mills, are devices used to rapidly grind materials to colloidal fineness (approximately 1 μm and below) by developing high grinding energy via centrifugal and/or planetary action.
beryllium → berilij
Beryllium was discovered by Friedrich Wöhler (Germany) and independently by A. B. Bussy (France) in 1828. The origin of the name comes from the Greek word beryllos meaning mineral beryl; also called glucinium from the Greek word glykys meaning sweet. It is steel-grey metal. It resists attack by concentrated nitric acid, has excellent thermal conductivity and is nonmagnetic. At ordinary temperatures, it resists oxidation in air. Beryllium and its salts are toxic and should be handled with the greatest of care. Beryllium is found mostly in minerals like beryl [AlBe3(Si6O18)] and chrysoberyl (Al2BeO4). Pure beryllium is obtained by chemically reducing beryl mineral. Also by electrolysis of beryllium chloride. Its ability to absorb large amounts of heat makes it useful in spacecraft, missiles, aircraft, etc. Emeralds are beryl crystals with chromium traces giving them their green colour.
Citing this page:
Generalic, Eni. "Elektroda drugog reda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table