electrochemical cell → elektrokemijski članak
Electrochemical cell is a device that converts chemical energy into electrical energy or vice versa when a chemical reaction is occurring in the cell. It consist of two electronically conducting phases (e.g., solid or liquid metals, semiconductors, etc) connected by an ionically conducting phase (e.g. aqueous or non-aqueous solution, molten salt, ionically conducting solid). As an electric current passes, it must change from electronic current to ionic current and back to electronic current. These changes of conduction mode are always accompanied by oxidation/reduction reactions.
An essential feature of the electrochemical cell is that the simultaneously occurring oxidation-reduction reactions are spatially separated. E.g., in a spontaneous chemical reaction during the oxidation of hydrogen by oxygen to water, electrons are passed directly from the hydrogen to the oxygen.
In contrast, in the spontaneous electrochemical reaction in a galvanic cell the hydrogen is oxidised at the anode by transferring electrons to the anode and the oxygen is reduced at the cathode by accepting electrons from the cathode. The ions produced in the electrode reactions, in this case positive hydrogen ions and the negative hydroxyl (OH-) ions, will recombine in the solution to form the final product of the reaction: water. During this process the electrons are conducted from the anode to the cathode through an outside electric circuit where the electric current can drive a motor, light a light bulb, etc. The reaction can also be reversed: water can be decomposed into hydrogen and oxygen by the application of electrical power in an electrolytic cell.
electrodialysis → elektrodijaliza
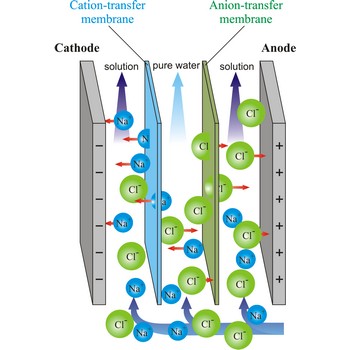
Electrodialysis is a procedure of dialysis accelerated with an electric field. Dialyser is divided into three sections. Solution flows through the middle section, between two semipermeable membranes alternately to positive ions and negative ions. An electrodes are placed in the neighbouring sections. Under the influence of electric field, positive ions will travel towards the cathode (the negative electrode), and negative ions towards the anode (the positive electrode), whereby travelling of ions through the membrane is accelerated. In this way, the feed water is separated into two streams: one of pure water and the other of more concentrated solution.
nonelectrolyte → neelektrolit
Nonelectrolytes are substances which in solutions do not dissociate into ions and they do not conduct electric current.
ohm → om
Ohm (Ω) is the SI derived unit of electric resistance. The ohm is the electric resistance between two points of a conductor when a constant difference of potential of one volt, applied between these two points, produces in this conductor a current of one ampere, this conductor not being the source of electromotive force (Ω = V/A). The unit was named after the German physicist Georg Simon Ohm (1789-1854).
electrolytes → elektroliti
Electrolytes are substances which, when melted or dissolved in water, conduct electric current. By melting or dissolving they are dissociated into electrically charged particles (ions) which are able to conduct electric current. By passing of electric current the transfer of matter occurs. Positively charged particles (cations) travel towards the negative pole (the cathode) and negatively charged particles (the anions) travel towards the positive pole (the anode). Liquid metals, in which the conduction is by free electrons, are not usually regarded as electrolytes. Solid conductors of ions, as in the sodium-sulphur cell, are also known as electrolytes. Depending upon how it conducts electric current, matter can be divided into strong electrolytes, weak electrolytes and nonconductors.
electrolytic cell → elektrolitska ćelija
Electrolytic cell is an electrochemical cell that converts electrical energy into chemical energy. The chemical reactions do not occur spontaneously at the electrodes when they are connected through an external circuit. The reaction must be forced by applying an external electric current. It is used to store electrical energy in chemical form (rechargeable battery). It is also used to decompose or produce (synthesise) new chemicals by the application of electrical power. This process is called electrolysis, e.g., water can be decomposed into hydrogen gas and oxygen gas. The free energy change of the overall cell reaction is positive.
electrophoresis → elektroforeza
Electrophoresis is a technique for the analysis and separation of colloids, based on the movement of charged colloidal particles in an electric field. The migration is toward electrodes of charge opposite to that of the particles. The rate of migration of the particles depends on the field, the charge on the particles, and on other factors, such as the size and shape of the particles.
Electrophoresis is important in the study of proteins. The acidity of the solution can be used to control the direction in which a protein moves upon electrophoresis.
Peltier effect → Peltierov efekt
Peltier effect is the absorption or generation of heat (depending on the current direction) which occurs when an electric current is passed through a junction between two materials.
Citing this page:
Generalic, Eni. "Električni dvosloj." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table