Hooke’s law → Hookeov zakon
Hooke’s law stating that the deformation of a body is proportional to the magnitude of the deforming force, provided that the body’s elastic limit (see elasticity) is not exceeded. If the elastic limit is not reached, the body will return to its original size once the force is removed. The law was discovered by English physicist Robert Hooke in 1676.
If a body on elastic spring is displaced from its equilibrium position (i.e. if the spring is stretched or compressed), a restitution force tries to return the body back in its equilibrium position. The magnitude of that force is proportional to the displacement of the body
Where F is the restitutional (elastic) force, x is the displacement of the body and k is the spring constant, which depends on dimensions, shape and material of the spring.
ion-product constant → ionski produkt vode
The ion-product constant. For the reaction:
the equilibrium expression would be:
Note that all pure liquid terms are omitted, hence H2O does not appear in the denominator. At 25 °C
lactose → laktoza
Lactose (milk sugar) is a disaccharide comprising one glucose molecule linked to a galactose molecule by an β(1→4)-glycosidic linkage. Lactose has a beta acetal. Lactose is manufactured by the mammary gland and occurs only in milk (from 4 % to 7 %). Lactose intolerance is a common medical condition that results in diarrhea, abdominal pain, and flatulence and is caused by reduced or absent activity of enzyme lactase.
Like cellobiose and maltose, lactose is a reducing sugar. All reducing sugar undergo mutarotation in aqueous solution. The equilibrium mixture at 20 °C is composed of 62.7 % β-lactose (β-D-galactopyranosyl-(1→4)-β-D-glucopyranose) and 37.3 % α-lactose (β-D-galactopyranosyl-(1→4)-α-D-glucopyranose).
melting point → talište
Melting point is the temperature at which a solid becomes a liquid at normal atmospheric pressure.
A more specific definition of melting point (or freezing point) is the temperature at which the solid and liquid phases of a substance are in equilibrium at a specified pressure (normally taken to be atmospheric unless stated otherwise). A pure substance under standard condition of pressure has a single reproducible melting point. The terms melting point and freezing point are often used interchangeably, depending on whether the substance is being heated or cooled.
mutarotation → mutarotacija
Mutarotation is the change in optical rotation accompanying epimerization. In carbohydrate chemistry this term usually refers to epimerization at the hemiacetal carbon atom. In general α- and β-form are stable solids, but in solution they rapidly equilibrate. For example, D-glucose exists in an equilibrium mixture of 36 % α-D-glucopyranose and 64 % β-D-glucopyranose, with only a tiny fraction in the open-chain form. The equilibration occurs via the ring opening of the cyclic sugar at the anomeric center with the acyclic form as the intermediate. Mutarotation was discovered by French chemist Augustin-Pierre Dubrunfaut (1797-1881) in 1846.
osmotic pressure → osmotski tlak
Osmotic pressure (Π) is the excess pressure necessary to maintain osmotic equilibrium between a solution and a pure solvent separated by a membrane permeable only to the solvent. In an ideal dilute solution
where cB is the amount-of-substance concentration of the solute, R is the molar gas constant, and T the temperature.
Ostwald’s dilution law → Ostwaldov zakon razrjeđenja
Ostwald’s dilution law is a relation for the concentration dependence of the molar conductivity Λ of an electrolyte solution, viz.
where c is the solute concentration, Kc is the equilibrium constant for dissociation of the solute, and L0 is the conductivity at cΛ = 0. The law was first put forward by the German chemist Wilhelm Ostwald (1853-1932).
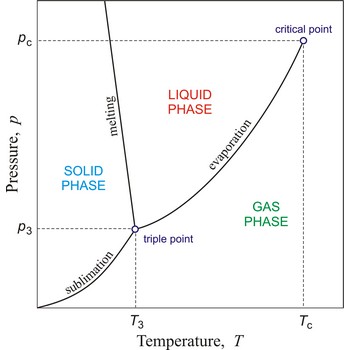
phase diagram → fazni dijagram
Phase diagram is a graphic representation of the equilibrium relationships between phases (such as vapour-liquid, liquid-solid) of a chemical compound, mixture of compounds, or solution.
The figure shows a typical phase diagram of an element or a simple compound. The stability of solid, liquid and gas phases depends on the temperature and the pressure. The three phases are in equilibrium at the triple point. The gas and liquid phases are separated by a phase transition only below the temperature of the critical point.
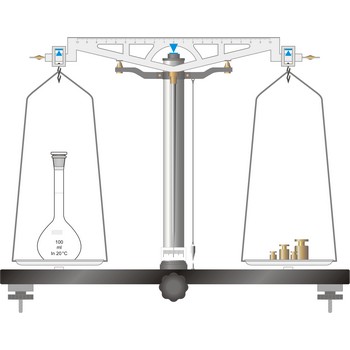
precision balance → tehnička vaga
Precision balances typically display results from three to one decimal places (0.001 g up to 0.1 g). The readability precision balances are reduced when compared to analytical balances but, precision balances accommodate higher capacities (up to several kilograms). In its traditional form, it consists of a pivoted horizontal lever of equal length arms, called the beam, with a weighing pan, also called scale, suspended from each arm.
In electronic top pan, or toploader balances, mass is determined not by mechanical deflection but by electronically controlled compensation of an electric force. The signal generated enables the mass to be read from a digital display. The mass of the empty container can be stored in the balance’s computer memory and automatically deducted from the mass of the container plus its contents.
reversible reaction → reverzibilna reakcija
Reversible reaction is a chemical reaction that can proceed in both the forward and backward directions. When reversible reactions reach equilibrium the forward and reverse reactions are still happening but at the same rate, so the concentrations of reactants and products do not change. A reversible reaction is denoted by a double arrow pointing both directions in a chemical equation.
Citing this page:
Generalic, Eni. "Dinamička ravnoteža." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table