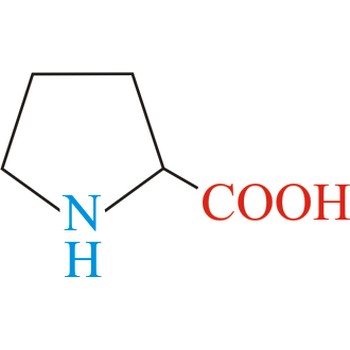
proline → prolin
Proline has an aliphatic side chain with a distinctive cyclic structure. It is unusual because it is conformationally restricted. The secondary amino (imino) group of proline residues is held in a rigid conformation that reduces the structural flexibility of polypeptide regions containing proline. It is not an essential amino acid, which means that the human body can synthesize it.
- Abbreviations: Pro, P
- IUPAC name: pyrrolidine-2-carboxylic acid
- Molecular formula: C5H9NO2
- Molecular weight: 115.13 g/mol
Rayleigh number → Rayleighova značajka
Rayleigh number (Ra) is a dimensionless quantity used in fluid mechanics, defined by
where l is length, g is acceleration of gravity, α is cubic expansion coefficient, T is temperature, ρ is density, η is viscosity, and a is thermal diffusivity.
resonance → rezonancija
Resonance is a stabilising quality of certain molecules that can be represented by considering the electron distribution in an ion or molecule as a composite of two or more forms, in those cases where a single form is an inadequate representation; for example, benzene and the carbonate ion. A various canonical structures can be drawn to show how electron delocalisation will explain the discrepancy, the difference in electron density
ribonucleic acid → ribonukleinska kiselina
Ribonucleic acid is a complex organic compound in living cells that is concerned with protein synthesis. Plays an intermediary role in converting the information contained in DNA into proteins. RNA carries the genetic information from DNA to those parts of the cell where proteins are made. Some viruses store their genetic information as RNA not as DNA.
Ribonucleic acid is a similar molecule to DNA but with a slightly different structure.
The structural difference with DNA is that RNA contains a -OH group both at the 2' and 3' position of the ribose ring, whereas DNA (which stands, in fact, for deoxy-RNA) lacks such a hydroxy group at the 2' position of the ribose. The same bases can be attached to the ribose group in RNA as occur in DNA, with the exception that in RNA thymine does not occur, and is replaced by uracil, which has an H-group instead of a methyl group at the C-5 position of the pyrimidine. Unlike the double-stranded DNA molecule, RNA is a single-stranded molecule.
The three main functionally distinct varieties of RNA molecules are: (1) messenger RNA (mRNA) which is involved in the transmission of DNA information, (2) ribosomal RNa (rRNA) which makes up the physical machinery of the synthetic process, and (3) transfer RNA (tRNA) which also constitutes another functional part of the machinery of protein synthesis.
scandium → skandij
Scandium was discovered by Lars Fredrik Nilson (Sweden) in 1879. The origin of the name comes from the Latin word Scandia meaning Scandinavia. It is fairly soft, silvery-white metal. Burns easily. Tarnishes readily in air. Reaction with water releases hydrogen. Reacts with air and halogens. Scandium occurs mainly in the minerals thortveitile (~34 % scandium) and wiikite. Also in some tin and tungsten ores. Pure scandium is obtained as a by-product of uranium refining. Scandium metal is used in some aerospace applications. Scandium oxide (Sc2O2) is used in the manufacture of high-intensity electric lamps. Scandium iodide (ScI3) is used in lamps that produce light having a colour closely matching natural sunlight.
sedimentary rocks → sedimentne stijene
Sedimentary Rocks are formed by the accumulation and subsequent consolidation of sediments into various types of rock. There are three major types of sedimentary rocks:
Biogenic sedimentary rocks are formed from organic processes when organisms use materials dissolved in water to build a shell or other skeletal structure.
Clastic sedimentary rocks are composed directly of the sediments or fragments from other rocks.
Chemical sedimentary rocks are formed through evaporation of a chemical rich solution.
Based on their sizes, sediment particles are classified, based on their size, into six general categories:
- boulder (>256 mm)
- cobble (64 - 256 mm)
- gravel (2 - 64 mm)
- sand (1/16 - 2 mm)
- silt (1/256 - 1/16 mm)
- clay (<1/256 mm)
solar cell → sunčeva ćelija
Solar cell, or photovoltaic cell, is a device that captures sunlight and transforms it directly to electricity. All solar cells make use of photovoltaic effect, so often they are called photovoltaic cells. Almost all solar cells are built from solid-state semiconducting materials, and in the vast majority of these the semiconductor is silicon.
The photovoltaic effect involves the generation of mobile charge carriers-electrons and positively charged holes-by the absorption of a photon of light. This pair of charge carriers is produced when an electron in the highest filled electronic band of a semiconductor (the valence band) absorbs a photon of sufficient energy to promote it into the empty energy band (the conduction band). The excitation process can be induced only by a photon with an energy corresponding to the width of the energy gap that separates the valence and the conduction band. The creation of an electron-hole pair can be converted into the generation of an electrical current in a semiconductor junction device, wherein a layer of semiconducting material lies back to back with a layer of either a different semiconductor or a metal. In most photovoltaic cells, the junction is p-n junction, in which p-doped and n-doped semiconductors are married together. At the interface of the two, the predominance of positively charged carriers (holes) in the p-doped material and of negatively charged carriers (electrons) in the n-doped material sets up an electric field, which falls off to either side of the junction across a space-charge region. When absorption of a photon in this region generates an electron-hole pair, these charge carriers are driven in opposite directions by the electric field, i.e. away from the interface and toward the top and bottom of the two-layer structure, where metal electrodes on these faces collect the current. The electrode on the top layer (through which light is absorbed) is divided into strips so as not to obscure the semiconducting layers below. In most widely used commercial solar cells, the p-doped and n-doped semiconductive layers are formed within a monolithic piece of crystalline silicon. Silicon is able to absorb sunlight at those wavelengths at which it is most intense-from the near-infrared region (wavelengths of around 1200 nm) to the violet (around 350 nm).
solid state → čvrsto agregatno stanje
Solid state is characterised by a constant shape and volume. Particles are placed very close to one another and have efect one on another with great attraction forces. Solid bodies do not assume the shape of the container in which they are put.
standard → standard
Standards are materials containing a known concentration of an analyte. They provide a reference to determine unknown concentrations or to calibrate analytical instruments.
The accuracy of an analytical measurement is how close a result comes to the true value. Determining the accuracy of a measurement usually requires calibration of the analytical method with a known standard. This is often done with standards of several concentrations to make a calibration or working curve.
A primary standard is a reagent that is extremely pure, stable, has no waters of hydration, and has a high molecular weight.
A secondary standard is a standard that is prepared in the laboratory for a specific analysis. It is usually standardised against a primary standard.
tantalum → tantal
Tantalum was discovered by Anders Ekeberg (Sweden) in 1802. The origin of the name comes from the Greek word Tantalos meaning father of Niobe in Greek mythology, (tantalum is closely related to niobium in the periodic table). It is rare, grey, heavy, hard but ductile, metal with a high melting point. Exposed surfaces form corrosion resistant oxide film. Attacked by HF and fused alkalis. Metal ignites in air. Tantalum always found with niobium. Chiefly occurs in the mineral tantalite. Often used as an economical substitute for platinum. Tantalum pentoxide is used in capacitors and in camera lenses to increase refracting power. It and its alloys are corrosion and wear resistant so it is used to make surgical and dental tools.
Citing this page:
Generalic, Eni. "Cubic close-packed structure." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table